Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, Graaf RD, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12:3–21.
PubMed
Google Scholar
Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–94.
PubMed
PubMed Central
Google Scholar
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547.
PubMed
PubMed Central
Google Scholar
Kennedy SH. Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin Neurosci. 2008;10:271–7.
PubMed
PubMed Central
Google Scholar
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
PubMed
Google Scholar
Coryell W, Young EA. Clinical predictors of suicide in primary major depressive disorder. J Clin Psychiatry. 2005;66:412–7.
PubMed
Google Scholar
Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–28.
PubMed
Google Scholar
van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1:63–72.
PubMed
Google Scholar
Dong M, Wang S-B, Li Y, Xu D-D, Ungvari GS, Ng CH, et al. Prevalence of suicidal behaviors in patients with major depressive disorder in China: a comprehensive meta-analysis. J Affect Disord. 2018;225:32–9.
PubMed
Google Scholar
Dong M, Zeng L-N, Lu L, Li X-H, Ungvari GS, Ng CH, et al. Prevalence of suicide attempt in individuals with major depressive disorder: a meta-analysis of observational surveys. Psychol Med. 2019;49:1691–704.
PubMed
Google Scholar
Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 2024;9:30.
PubMed
PubMed Central
Google Scholar
Pompili M, Venturini P, Palermo M, Stefani H, Seretti ME, Lamis DA, et al. Mood disorders medications: predictors of nonadherence – review of the current literature. Expert Rev Neurother. 2013;13:809–25.
PubMed
Google Scholar
Mariani N, Cattane N, Pariante C, Cattaneo A. Gene expression studies in depression development and treatment: an overview of the underlying molecular mechanisms and biological processes to identify biomarkers. Transl Psychiatry. 2021;11:354.
PubMed
PubMed Central
Google Scholar
Manzoni C, Kia DA, Vandrovcova J, Hardy J, Wood NW, Lewis PA, et al. Genome, transcriptome and proteome: the rise of omics data and their integration in biomedical sciences. Brief Bioinform. 2018;19:286–302.
PubMed
Google Scholar
Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:1–15.
Google Scholar
Niu X, Zhang J, Zhang L, Hou Y, Pu S, Chu A, et al. Weighted gene co-expression network analysis identifies critical genes in the development of heart failure after acute myocardial infarction. Front Genet. 2019;10:1214.
PubMed
PubMed Central
Google Scholar
Bouzid A, Almidani A, Zubrikhina M, Kamzanova A, Ilce BY, Zholdassova M, et al. Integrative bioinformatics and artificial intelligence analyses of transcriptomics data identified genes associated with major depressive disorders including NRG1. Neurobiol Stress. 2023;26:100555.
PubMed
PubMed Central
Google Scholar
Nickson D, Meyer C, Walasek L, Toro C. Prediction and diagnosis of depression using machine learning with electronic health records data: a systematic review. BMC Med Inform Decis Mak. 2023;23:271.
PubMed
PubMed Central
Google Scholar
Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63.
PubMed
PubMed Central
Google Scholar
Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50:1–14.
PubMed
PubMed Central
Google Scholar
Liang M, Cowley JrAW, Greene AS. High throughput gene expression profiling: a molecular approach to integrative physiology. J Physiol. 2004;554:22–30.
PubMed
Google Scholar
Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, et al. A molecular signature of depression in the amygdala. Am J psychiatry. 2009;166:1011–24.
PubMed
PubMed Central
Google Scholar
Cruceanu C, Tan PPC, Rogic S, Lopez JP, Torres-Platas SG, Gigek CO, et al. Transcriptome sequencing of the anterior cingulate in bipolar disorder: dysregulation of G protein-coupled receptors. Am J psychiatry. 2015;172:1131–40.
PubMed
Google Scholar
Zhou Y, Lutz P-E, Wang YC, Ragoussis J, Turecki G. Global long non-coding RNA expression in the rostral anterior cingulate cortex of depressed suicides. Transl Psychiatry. 2018;8:224.
PubMed
PubMed Central
Google Scholar
Kim B, Kim D, Schulmann A, Patel Y, Caban-Rivera C, Kim P, et al. Cellular diversity in human subgenual anterior cingulate and dorsolateral prefrontal cortex by single-nucleus RNA-sequencing. J Neurosci. 2023;43:3582–97.
PubMed
PubMed Central
Google Scholar
Pantazatos SP, Huang Y, Rosoklija GB, Dwork AJ, Arango V, Mann JJ. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry. 2017;22:760–73.
PubMed
Google Scholar
Yoshino Y, Roy B, Kumar N, Shahid Mukhtar M, Dwivedi Y. Molecular pathology associated with altered synaptic transcriptome in the dorsolateral prefrontal cortex of depressed subjects. Transl Psychiatry. 2021;11:73.
PubMed
PubMed Central
Google Scholar
Dóra F, Renner É, Keller D, Palkovits M, Dobolyi Á. Transcriptome profiling of the dorsomedial prefrontal cortex in suicide victims. Int J Mol Sci. 2022;23:7067.
PubMed
PubMed Central
Google Scholar
Mahajan GJ, Vallender EJ, Garrett MR, Challagundla L, Overholser JC, Jurjus G, et al. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:177–86.
PubMed
Google Scholar
Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron. 2016;90:969–83.
PubMed
PubMed Central
Google Scholar
Mehta D, Menke A, Binder EB. Gene expression studies in major depression. Curr Psychiatry Rep. 2010;12:135–44.
PubMed
PubMed Central
Google Scholar
Han M, Yuan L, Huang Y, Wang G, Du C, Wang Q, et al. Integrated co-expression network analysis uncovers novel tissue-specific genes in major depressive disorder and bipolar disorder. Front Psychiatry. 2022;13:980315.
PubMed
PubMed Central
Google Scholar
Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–11.
PubMed
PubMed Central
Google Scholar
Mansouri S, Pessoni AM, Marroquín-Rivera A, Parise EM, Tamminga CA, Turecki G, et al. Transcriptional dissection of symptomatic profiles across the brain of men and women with depression. Nat Commun. 2023;14:6835.
PubMed
PubMed Central
Google Scholar
Sun S, Liu Q, Wang Z, Huang Y-y, Sublette ME, Dwork AJ, et al. Brain and blood transcriptome profiles delineate common genetic pathways across suicidal ideation and suicide. Mol Psychiatry. 2024;29:1417–26.
PubMed
PubMed Central
Google Scholar
Seney ML, Glausier J, Sibille E. Large-scale transcriptomics studies provide insight into sex differences in depression. Biol Psychiatry. 2022;91:14–24.
PubMed
Google Scholar
Liew C-C, Ma J, Tang H-C, Zheng R, Dempsey AA. The peripheral blood transcriptome dynamically reflects system wide biology: a potential diagnostic tool. J Lab Clin Med. 2006;147:126–32.
PubMed
Google Scholar
Harrington CA, Fei SS, Minnier J, Carbone L, Searles R, Davis BA, et al. RNA-Seq of human whole blood: Evaluation of globin RNA depletion on Ribo-Zero library method. Sci Rep. 2020;10:6271.
PubMed
PubMed Central
Google Scholar
Hennings J, Uhr M, Klengel T, Weber P, Pütz B, Touma C, et al. RNA expression profiling in depressed patients suggests retinoid-related orphan receptor alpha as a biomarker for antidepressant response. Transl Psychiatry. 2015;5:e538–e538.
PubMed
PubMed Central
Google Scholar
Ju C, Fiori LM, Belzeaux R, Theroux J-F, Chen GG, Aouabed Z, et al. Integrated genome-wide methylation and expression analyses reveal functional predictors of response to antidepressants. Transl Psychiatry. 2019;9:254.
PubMed
PubMed Central
Google Scholar
Le-Niculescu H, Kurian S, Yehyawi N, Dike C, Patel S, Edenberg H, et al. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry. 2009;14:156–74.
PubMed
Google Scholar
Le-Niculescu H, Levey D, Ayalew M, Palmer L, Gavrin L, Jain N, et al. Discovery and validation of blood biomarkers for suicidality. Mol Psychiatry. 2013;18:1249–64.
PubMed
PubMed Central
Google Scholar
Woo HI, Lim S-W, Myung W, Kim DK, Lee S-Y. Differentially expressed genes related to major depressive disorder and antidepressant response: genome-wide gene expression analysis. Exp Mol Med. 2018;50:1–11.
PubMed
PubMed Central
Google Scholar
Geng R, Li Z, Yu S, Yuan C, Hong W, Wang Z, et al. Weighted gene co-expression network analysis identifies specific modules and hub genes related to subsyndromal symptomatic depression. World J Biol Psychiatry. 2020;21:102–10.
PubMed
Google Scholar
Wang Q, Timberlake IIMA, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:99–109.
PubMed
PubMed Central
Google Scholar
Wang Q, Dwivedi Y. Advances in novel molecular targets for antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110041.
PubMed
Google Scholar
Peng S, Zhou Y, Lu M, Wang Q. Review of herbal medicines for the treatment of depression. Nat Prod Commun. 2022;17:1934578X221139082.
Google Scholar
Roy B, Wang Q, Dwivedi Y. Long noncoding RNA-associated transcriptomic changes in resiliency or susceptibility to depression and response to antidepressant treatment. Int J Neuropsychopharmacol. 2018;21:461–72.
PubMed
PubMed Central
Google Scholar
Svenningsen K, Venø MT, Henningsen K, Mallien AS, Jensen L, Christensen T, et al. MicroRNA profiling in the medial and lateral habenula of rats exposed to the learned helplessness paradigm: candidate biomarkers for susceptibility and resilience to inescapable shock. PLoS ONE. 2016;11:e0160318.
PubMed
PubMed Central
Google Scholar
Gui S, Liu Y, Pu J, Song X, Chen X, Chen W, et al. Comparative analysis of hippocampal transcriptional features between major depressive disorder patients and animal models. J Affect Disord. 2021;293:19–28.
PubMed
Google Scholar
Bondar N, Bryzgalov L, Ershov N, Gusev F, Reshetnikov V, Avgustinovich D, et al. Molecular adaptations to social defeat stress and induced depression in mice. Mol Neurobiol. 2018;55:3394–407.
PubMed
Google Scholar
Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356:1185–8.
PubMed
PubMed Central
Google Scholar
Peña CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun. 2019;10:5098.
PubMed
PubMed Central
Google Scholar
Wang Q, Roy B, Dwivedi Y. Co-expression network modeling identifies key long non-coding RNA and mRNA modules in altering molecular phenotype to develop stress-induced depression in rats. Transl Psychiatry. 2019;9:125.
PubMed
PubMed Central
Google Scholar
Lei C, Li N, Chen J, Wang Q. Hypericin ameliorates depression-like behaviors via neurotrophin signaling pathway mediating m6a epitranscriptome modification. Molecules. 2023;28:3859.
PubMed
PubMed Central
Google Scholar
Hing B, Mitchell S, Eberle M, Filali Y, Matkovich M, Kasturirangan M. et al. Transcriptomic evaluation of stressvulnerability network using single cell RNA-seq in mouse prefrontal cortex. Biol Psychiatry. 2024;96:866–99.
Google Scholar
Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–65.
PubMed
PubMed Central
Google Scholar
Bartel DP. Metazoan micrornas. Cell. 2018;173:20–51.
PubMed
PubMed Central
Google Scholar
Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192:98–105.
PubMed
PubMed Central
Google Scholar
Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–43.
PubMed
Google Scholar
Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS ONE. 2012;7:e33201.
PubMed
PubMed Central
Google Scholar
Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS ONE. 2014;9:e86469.
PubMed
PubMed Central
Google Scholar
Roy B, Wang Q, Palkovits M, Faludi G, Dwivedi Y. Altered miRNA expression network in locus coeruleus of depressed suicide subjects. Sci Rep. 2017;7:4387.
PubMed
PubMed Central
Google Scholar
Yoshino Y, Roy B, Dwivedi Y. Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects. Neuropsychopharmacology. 2021;46:900–10.
PubMed
Google Scholar
Yoshino Y, Roy B, Dwivedi Y. Altered miRNA landscape of the anterior cingulate cortex is associated with potential loss of key neuronal functions in depressed brain. Eur Neuropsychopharmacol. 2020;40:70–84.
PubMed
Google Scholar
Roy B, Dunbar M, Shelton RC, Dwivedi Y. Identification of MicroRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology. 2017;42:864–75.
PubMed
Google Scholar
Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y. Role of complex epigenetic switching in tumor necrosis factor-α upregulation in the prefrontal cortex of suicide subjects. Am J Psychiatry. 2018;175:262–74.
PubMed
PubMed Central
Google Scholar
Smalheiser NR, Lugli G, Rizavi HS, Zhang H, Torvik VI, Pandey GN, et al. MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. Int J Neuropsychopharmacol. 2011;14:1315–25.
PubMed
Google Scholar
McKibben LA, Dwivedi Y. Early-life stress induces genome-wide sex-dependent miRNA expression and correlation across limbic brain areas in rats. Epigenomics. 2021;13:1031–56.
PubMed
PubMed Central
Google Scholar
McKibben LA, Dwivedi Y. Early life and adult stress promote sex dependent changes in hypothalamic miRNAs and environmental enrichment prevents stress-induced miRNA and gene expression changes in rats. BMC Genomics. 2021;22:701.
PubMed
PubMed Central
Google Scholar
McKibben LA, Dwivedi Y. MicroRNA regulates early-life stress-induced depressive behavior via serotonin signaling in a sex-dependent manner in the prefrontal cortex of rats. Biol Psychiatry Glob Open Sci. 2021;1:180–9.
PubMed
PubMed Central
Google Scholar
Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med. 2014;20:764–8.
PubMed
PubMed Central
Google Scholar
Lopez JP, Fiori LM, Gross JA, Labonte B, Yerko V, Mechawar N, et al. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int J Neuropsychopharmacol. 2014;17:23–32.
PubMed
Google Scholar
Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat Commun. 2017;8:15497.
PubMed
PubMed Central
Google Scholar
Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, et al. Investigation of miR-1202, miR-135a, and miR-16 in major depressive disorder and antidepressant response. Int J Neuropsychopharmacol. 2017;20:619–23.
PubMed
PubMed Central
Google Scholar
Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen L-L, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol. 2023;24:430–47.
PubMed
PubMed Central
Google Scholar
Statello L, Guo C-J, Chen L-L, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118.
PubMed
Google Scholar
Karlsson O, Baccarelli AA. Environmental health and long non-coding RNAs. Curr Environ Health Rep. 2016;3:178–87.
PubMed
PubMed Central
Google Scholar
Zimmer-Bensch G. Emerging roles of long non-coding RNAs as drivers of brain evolution. Cells. 2019;8:1399.
PubMed
PubMed Central
Google Scholar
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14.
PubMed
PubMed Central
Google Scholar
Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, et al. Sex-specific role for the long non-coding RNA LINC00473 in depression. Neuron. 2020;106:912–26.e915.
PubMed
PubMed Central
Google Scholar
Cui X, Niu W, Kong L, He M, Jiang K, Chen S, et al. Long noncoding RNA expression in peripheral blood mononuclear cells and suicide risk in Chinese patients with major depressive disorder. Brain Behav. 2017;7:e00711.
PubMed
PubMed Central
Google Scholar
Issler O, van der Zee YY, Ramakrishnan A, Xia S, Zinsmaier AK, Tan C, et al. The long noncoding RNA FEDORA is a cell type–and sex-specific regulator of depression. Sci Adv. 2022;8:eabn9494.
PubMed
PubMed Central
Google Scholar
Punzi G, Ursini G, Viscanti G, Radulescu E, Shin JH, Quarto T, et al. Association of a noncoding RNA postmortem with suicide by violent means and in vivo with aggressive phenotypes. Biol Psychiatry. 2019;85:417–24.
PubMed
Google Scholar
Wang Q, Wang H, Dwivedi Y. Integrated long noncoding RNA and messenger RNA expression analysis identifies molecules specifically associated with resiliency and susceptibility to depression and antidepressant response. Biol Psychiatry Glob Open Sci. 2024;4:100365.
PubMed
PubMed Central
Google Scholar
Xie R, Zhang Y, Zhang J, Li J, Zhou X. The role of circular RNAs in immune-related diseases. Front Immunol. 2020;11:545.
PubMed
PubMed Central
Google Scholar
Yu C-Y, Kuo H-C. The emerging roles and functions of circular RNAs and their generation. J Biomed Sci. 2019;26:29.
PubMed
PubMed Central
Google Scholar
Shi Y, Wang Q, Song R, Kong Y, Zhang Z. Non-coding RNAs in depression: Promising diagnostic and therapeutic biomarkers. EBioMedicine. 2021;71:103569.
PubMed
PubMed Central
Google Scholar
Zimmerman AJ, Hafez AK, Amoah SK, Rodriguez BA, Dell’Orco M, Lozano E, et al. A psychiatric disease-related circular RNA controls synaptic gene expression and cognition. Mol Psychiatry. 2020;25:2712–27.
PubMed
PubMed Central
Google Scholar
Cui X, Niu W, Kong L, He M, Jiang K, Chen S, et al. hsa_circRNA_103636: potential novel diagnostic and therapeutic biomarker in Major depressive disorder. Biomark Med. 2016;10:943–52.
PubMed
Google Scholar
Shi Y, Song R, Wang Z, Zhang H, Zhu J, Yue Y, et al. Potential clinical value of circular RNAs as peripheral biomarkers for the diagnosis and treatment of major depressive disorder. EBioMedicine. 2021;66:103337.
PubMed
PubMed Central
Google Scholar
Huang Z-h, Du Y-p, Wen J-t, Lu B-f, Zhao Y. snoRNAs: functions and mechanisms in biological processes, and roles in tumor pathophysiology. Cell Death Discov. 2022;8:259.
PubMed
PubMed Central
Google Scholar
Wajahat M, Bracken CP, Orang A. Emerging functions for snoRNAs and snoRNA-derived fragments. Int J Mol Sci. 2021;22:10193.
PubMed
PubMed Central
Google Scholar
Lin R, Kos A, Lopez JP, Dine J, Fiori LM, Yang J, et al. SNORD90 induces glutamatergic signaling following treatment with monoaminergic antidepressants. eLife. 2023;12:e85316.
PubMed
PubMed Central
Google Scholar
Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–7.
PubMed
Google Scholar
Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202.
PubMed
Google Scholar
Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12:246–58.
PubMed
Google Scholar
Weick E-M, Miska EA. piRNAs: from biogenesis to function. Development. 2014;141:3458–71.
PubMed
Google Scholar
Sato K, Takayama K-i, Inoue S. Role of piRNA biogenesis and its neuronal function in the development of neurodegenerative diseases. Front Aging Neurosci. 2023;15:1157818.
PubMed
PubMed Central
Google Scholar
Nandi S, Chandramohan D, Fioriti L, Melnick AM, Hébert JM, Mason CE, et al. Roles for small noncoding RNAs in silencing of retrotransposons in the mammalian brain. Proc Natl Acad Sci USA. 2016;113:12697–702.
PubMed
PubMed Central
Google Scholar
Piwecka M, Rajewsky N, Rybak-Wolf A. Single-cell and spatial transcriptomics: deciphering brain complexity in health and disease. Nat Rev Neurol. 2023;19:346–62.
PubMed
PubMed Central
Google Scholar
Kashima Y, Sakamoto Y, Kaneko K, Seki M, Suzuki Y, Suzuki A. Single-cell sequencing techniques from individual to multiomics analyses. Exp Mol Med. 2020;52:1419–27.
PubMed
PubMed Central
Google Scholar
Liao J, Lu X, Shao X, Zhu L, Fan X. Uncovering an organ’s molecular architecture at single-cell resolution by spatially resolved transcriptomics. Trends Biotechnol. 2021;39:43–58.
PubMed
Google Scholar
Chen H, Albergante L, Hsu JY, Lareau CA, Lo Bosco G, Guan J, et al. Single-cell trajectories reconstruction, exploration and mapping of omics data with STREAM. Nat Commun. 2019;10:1903.
PubMed
PubMed Central
Google Scholar
Cuevas-Diaz Duran R, González-Orozco JC, Velasco I, Wu JQ. Single-cell and single-nuclei RNA sequencing as powerful tools to decipher cellular heterogeneity and dysregulation in neurodegenerative diseases. Front Cell Dev Biol. 2022;10:884748.
PubMed
PubMed Central
Google Scholar
Titus AJ, Gallimore RM, Salas LA, Christensen BC. Cell-type deconvolution from DNA methylation: a review of recent applications. Hum Mol Genet. 2017;26:R216–r224.
PubMed
PubMed Central
Google Scholar
Momeni K, Ghorbian S, Ahmadpour E, Sharifi R. Unraveling the complexity: understanding the deconvolutions of RNA-seq data. Transl Med Commun. 2023;8:21.
Google Scholar
Maitra M, Mitsuhashi H, Rahimian R, Chawla A, Yang J, Fiori LM, et al. Cell type specific transcriptomic differences in depression show similar patterns between males and females but implicate distinct cell types and genes. Nat Commun. 2023;14:2912.
PubMed
PubMed Central
Google Scholar
Zeng L, Fujita M, Gao Z, White CC, Green GS, Habib N. et al. A single-nucleus transcriptome-wide association studyimplicates novel genes in depression pathogenesis. Biol Psychiatry. 2024;96:34–43.
PubMed
Google Scholar
von Ziegler LM, Floriou-Servou A, Waag R, Das Gupta RR, Sturman O, Gapp K, et al. Multiomic profiling of the acute stress response in the mouse hippocampus. Nat Commun. 2022;13:1824.
Google Scholar
Pinu FR, Beale DJ, Paten AM, Kouremenos K, Swarup S, Schirra HJ, et al. Systems biology and multi-omics integration: viewpoints from the metabolomics research community. Metabolites. 2019;9:76.
PubMed
PubMed Central
Google Scholar
Mokhtari A, Porte B, Belzeaux R, Etain B, Marie-Claire C, Lutz P-E, et al. The molecular pathophysiology of mood disorders: from the analysis of single molecular layers to multi-omic integration. Prog Neuropsychopharmacol Biol Psychiatry. 2022;116:110520.
PubMed
Google Scholar
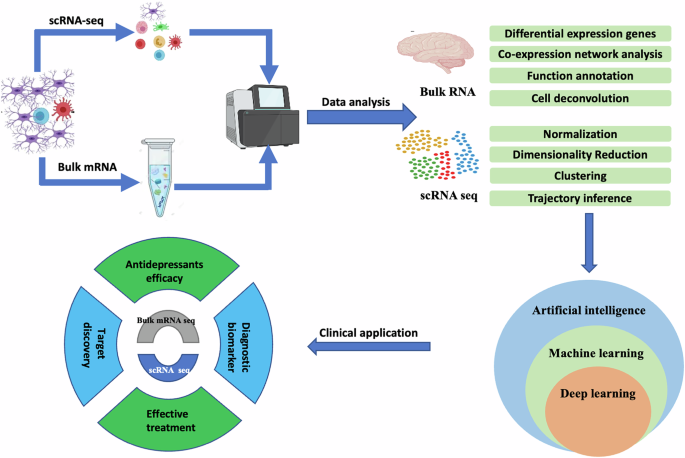
Zhou Y, Xiong L, Chen J, Wang Q. Integrative analyses of scRNA-seq, bulk mRNA-seq, and DNA methylation profiling in depressed suicide brain tissues. Int J Neuropsychopharmacol. 2023;26:840–55.
PubMed
PubMed Central
Google Scholar
Yi Z, Li Z, Yu S, Yuan C, Hong W, Wang Z, et al. Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PLoS ONE. 2012;7:e31283.
PubMed
PubMed Central
Google Scholar
Yu J, Xue A, Redei E, Bagheri N. A support vector machine model provides an accurate transcript-level-based diagnostic for major depressive disorder. Transl Psychiatry. 2016;6:e931–e931.
PubMed
PubMed Central
Google Scholar
Bhak Y, Jeong HO, Cho YS, Jeon S, Cho J, Gim JA, et al. Depression and suicide risk prediction models using blood-derived multi-omics data. Transl Psychiatry. 2019;9:262.
PubMed
PubMed Central
Google Scholar
Qi B, Ramamurthy J, Bennani I, Trakadis YJ. Machine learning and bioinformatic analysis of brain and blood mRNA profiles in major depressive disorder: a case–control study. Am J Med Genet B Neuropsychiatr Genet. 2021;186:101–12.
PubMed
Google Scholar
Zhang J, Kaye AP, Wang J, Girgenti MJ. Transcriptomics of the depressed and PTSD brain. Neurobiol Stress. 2021;15:100408.
PubMed
PubMed Central
Google Scholar
Zhou Y, Lutz PE, Ibrahim EC, Courtet P, Tzavara E, Turecki G, et al. Suicide and suicide behaviors: a review of transcriptomics and multiomics studies in psychiatric disorders. J Neurosci Res. 2020;98:601–15.
PubMed
Google Scholar
Furczyk K, Schutová B, Michel TM, Thome J, Büttner A. The neurobiology of suicide-A review of post-mortem studies. J Mol Psychiatry. 2013;1:2.
PubMed
PubMed Central
Google Scholar
Barnes J, Mondelli V, Pariante CM. Genetic contributions of inflammation to depression. Neuropsychopharmacology. 2017;42:81–98.
PubMed
Google Scholar
Allen L, Dwivedi Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol Psychiatry. 2020;25:308–20.
PubMed
Google Scholar
Peng S, Zhou Y, Xiong L, Wang Q. Identification of novel targets and pathways to distinguish suicide dependent or independent on depression diagnosis. Sci Rep. 2023;13:2488.
PubMed
PubMed Central
Google Scholar
Qi B, Fiori LM, Turecki G, Trakadis YJ. Machine learning analysis of blood microRNA data in major depression: A case-control study for biomarker discovery. Int J Neuropsychopharmacol. 2020;23:505–10.
PubMed
PubMed Central
Google Scholar
Williams CG, Lee HJ, Asatsuma T, Vento-Tormo R, Haque A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022;14:68.
PubMed
PubMed Central
Google Scholar
Du J, Yang Y-C, An Z-J, Zhang M-H, Fu X-H, Huang Z-F, et al. Advances in spatial transcriptomics and related data analysis strategies. J Transl Med. 2023;21:330.
PubMed
PubMed Central
Google Scholar
Fang S, Chen B, Zhang Y, Sun H, Liu L, Liu S, et al. Computational approaches and challenges in spatial transcriptomics. Genom Prot Bioinform. 2023;21:24–47.
Google Scholar
Thompson JR, Nelson ED, Tippani M, Ramnauth AD, Divecha HR, Miller RA, et al. An integrated single-nucleus and spatial transcriptomics atlas reveals the molecular landscape of the human hippocampus. Nat Neurosci. 2025. https://doi.org/10.1101/2024.04.26.590643
Maynard KR, Collado-Torres L, Weber LM, Uytingco C, Barry BK, Williams SR, et al. Transcriptome-scale spatial gene expression in the human dorsolateral prefrontal cortex. Nat Neurosci. 2021;24:425–36.
PubMed
PubMed Central
Google Scholar
Bhattacherjee A, Zhang C, Watson BR, Djekidel MN, Moffitt JR, Zhang Y. Spatial transcriptomics reveals the distinct organization of mouse prefrontal cortex and neuronal subtypes regulating chronic pain. Nat Neurosci. 2023;26:1880–93.
PubMed
PubMed Central
Google Scholar
Yao Z, van Velthoven CTJ, Kunst M, Zhang M, McMillen D, Lee C, et al. A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature. 2023;624:317–32.
PubMed
PubMed Central
Google Scholar
Joglekar A, Prjibelski A, Mahfouz A, Collier P, Lin S, Schlusche AK, et al. A spatially resolved brain region- and cell type-specific isoform atlas of the postnatal mouse brain. Nat Commun. 2021;12:463.
PubMed
PubMed Central
Google Scholar
Cho WC. Proteomics technologies and challenges. Genom Prot Bioinform. 2007;5:77–85.
Google Scholar
Lee S, Vu HM, Lee JH, Lim H, Kim MS. Advances in mass spectrometry-based single cell analysis. Biology. 2023;12:395.
PubMed
PubMed Central
Google Scholar
Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell. 2020;183:1665–81.e1618.
PubMed
PubMed Central
Google Scholar
Sarker IH. Machine learning: algorithms, real-world applications and research directions. SN Comput Sci. 2021;2:160.
PubMed
PubMed Central
Google Scholar
Kocaman E, Erdem MK, Calis G. Machine learning thermal comfort prediction models based on occupant demographic characteristics. J Therm Biol. 2024;123:103884.
PubMed
Google Scholar
Sarica A, Cerasa A, Quattrone A. Random forest algorithm for the classification of neuroimaging data in Alzheimer’s disease: a systematic review. Front Aging Neurosci. 2017;9:329.
PubMed
PubMed Central
Google Scholar
Alzubaidi L, Zhang J, Humaidi AJ, Al-Dujaili A, Duan Y, Al-Shamma O, et al. Review of deep learning: concepts, CNN architectures, challenges, applications, future directions. J Big Data. 2021;8:53.
PubMed
PubMed Central
Google Scholar
Gusev A, Mancuso N, Won H, Kousi M, Finucane HK, Reshef Y, et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat Genet. 2018;50:538–48.
PubMed
PubMed Central
Google Scholar
Al Hanai T, Ghassemi M, Glass J. Detecting depression with audio/text sequence modeling of interviews. In: Proceedings of the Annual Conference of the International Speech Communication Association, INTERSPEECH. 9th Annual Conference of the International Speech Communication, INTERSPEECH 2018 – Hyderabad, India. 2018.
Walsh CG, Ribeiro JD, Franklin JC. Predicting suicide attempts in adolescents with longitudinal clinical data and machine learning. J Child Psychology Psychiatry. 2018;59:1261–70.
Google Scholar
Quaak M, van de Mortel L, Thomas RM, van Wingen G. Deep learning applications for the classification of psychiatric disorders using neuroimaging data: systematic review and meta-analysis. NeuroImage Clin. 2021;30:102584.
PubMed
PubMed Central
Google Scholar
Kreimeyer K, Foster M, Pandey A, Arya N, Halford G, Jones SF, et al. Natural language processing systems for capturing and standardizing unstructured clinical information: a systematic review. J Biomed Inform. 2017;73:14–29.
PubMed
PubMed Central
Google Scholar
Birnbaum ML, Abrami A, Heisig S, Ali A, Arenare E, Agurto C, et al. Acoustic and facial features from clinical interviews for machine learning-based psychiatric diagnosis: algorithm development. JMIR Ment Health. 2022;9:e24699.
PubMed
PubMed Central
Google Scholar