AI Research
Investigating the role of molecular coating in human corneal endothelial cell primary culture using artificial intelligence-driven image analysis
Donor tissue and ethical statement
hCECs used in this study were obtained from primary cell cultures derived from organ-cultured human corneas. They were sourced from corneal donations for scientific purposes (Laboratory of Anatomy of the Faculty of Medicine of Saint-Étienne) or from three French eye banks (Besançon, Saint-Étienne, and Nantes). All corneas were unsuitable for transplantation because their parameters did not meet the required standards for clinical use. All experimental protocols were approved by a designated institutional and/or licensing committee and informed consent were obtained from all subjects and/or their legal guardian(s). The BiiO laboratory, authorized by the Ministry of Higher Education, Research, and Innovation of France (Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation, MESRI) under the number DC-2023-5458, conducted this research on these human corneas without the need for additional ethical approval. They were handled in accordance with the principles of the Declaration of Helsinki, bioethics laws, and French and European regulations regarding tissue donations.
For AI fine-tuning, we used 15 immunofluorescence-labeled images from 3 different cultures (see and S13 and data in the supplementary section). For AI segmentation and criteria validation, we used 4 other different endothelial cell cultures (listed in Table 1) which had varying quality, ranging from a culture undergoing endothelial-mesenchymal transition to an endothelial culture from a very young donor (2 years old, characterized by high ECD and excellent morphology). For the comparison of coating molecules, eight cultures from eight donors were used. The donors ages ranged from 55 to 85 years, with a mean ± standard deviation of 71 ± 11 years (sex ratio = 1 (4 males, 4 females)) and corneas were stored at 31 °C in organ culture medium (CorneaMax, Eurobio, Les Ulis, France) during 25 ± 22 days (min = 4; max = 72) before use. The initial ECD, measured within 5 days after retrieval, was 2390 ± 559 (1287–3233) cells/mm2. Donors and cultures characteristics were detailed in Table 1.
Cell culture media
Three different media were used: 1/The DM digestion medium consisted of Opti-MEM (11058021, Gibco, Grand Island, NY, USA) supplemented with 200 µg/mL CaCl2 (C5670, Sigma-Aldrich, St. Louis, MO, USA); 20 µg/mL ascorbic acid (A5960, Sigma-Aldrich) and 2 mg/mL collagenase A (10103586001, Roche, Basel, Switzerland). 2/The basic medium consisted of Opti-MEM supplemented with antibiotic–antimycotic (152 − 062, Gibco) at 1/200, 8% of fetal bovine serum (S-1860-500, Eurobio), 0.1% of CaCl2, 0.08% of chondroitin sulfate (034-1462, FUJIFILM Wako Pure Chemical Corporation, San Diego, CA, USA), 10 µM of SB203580 (3.8 µg/mL) (S1076, SelleckChem, Houston, TX, USA) and 1 µM of SB431542 (S1067, SelleckChem) (where SB203580 was an inhibitor of the p38 MAPK pathway and SB431542 an inhibitor of the TGF-β receptor; both involved in the limitation of EndoMT in cell culture), 20 µg/mL of ascorbic acid and 5ng/mL of Epidermal Growth Factor (EGF) (PHG0311, Gibco, Grand Island, NY, USA). 3/The medium for the initiation of primary cell culture and for cell passaging was the basic medium with the addition of a ROCK inhibitor (RI), Y-27,632 (S1049, SelleckChem) to promote cell adherence, proliferation and survival17,18,19.
Primary culture of hCECs
hCECs were cultivated using a protocol derived from the standard peel and digest method, also used by the team which selected the coating with iMatrix-511 (892012, Nippi, Incorporated, Tokyo, Japan)20. We increased the digestion time from 12 to 16 h and used a higher collagenase concentration (from 1 mg/mL to 2 mg/mL) to enhance cell dissociation, as previously described21. To isolate hCECs, DM was mechanically peeled from donor corneas, ensuring maximum peripheral coverage. The membranes were then incubated at 37 °C in a humidified 5% CO₂ atmosphere for 16 h with 0.5 mL of digestion medium in 48-well plates (3548, Corning, Kennebunk, ME, USA). After digestion, released hCECs were centrifuged at 200 g for 5 min in conical-bottom tubes (641997, Dutscher, Caplugs Evergreen, CA, USA). The cells were then seeded in 24-well plates (3524, Corning) pre-coated with iMatrix-511 according to the manufacturer’s instructions. hCECs were cultured in a humidified 5% CO2 atmosphere at 37 °C. The basic medium was changed the day after seeding and then weekly until confluence, which took about a month.
For cell passage, hCECs cultures were first rinsed in Ca2+ and Mg2+-free phosphate buffered saline (PBS) (SH30256.02, Cytiva, MA, USA), then incubated in TrypLE Select Enzyme 5X (A1217701, Gibco) for 15 min at 37 °C until cell detachment. Cells were detached mechanically by aspirating and dispensing the liquid in the well and chemically using TrypLE solution. Cells were then transferred in conical-bottom tubes containing basic medium supplemented with RI. The complete detachment of cells was confirmed using a phase-contrast microscope (CKX41, Olympus, Tokyo, Japan). Viable cells were counted using Trypan blue staining and an automatic cell counter (TC10, Bio-Rad, Hercules, CA, USA). Counting was performed twice to ensure repeatability and to calculate the average cell number. Passage followed a defined scaling ratio: P1 (1 well of a 24-well plate into 1 well of a 12-well plate), and P2 (1 well of a 12-well plate into 1 well of a 6-well plate), with subsequent passages at a 1:2 ratio. Throughout all stages, cells used for experiments were cultured on plates coated with the reference molecule, iMatrix-511 until experiment with the different molecular coatings. Cultured hCECs at passages 4 through 9 were used for these experiments. After the last cell counting, hCECs were centrifuged at 200 g for 5 min. The supernatant was removed and hCECs were resuspended in the medium previously described and seeded, this time, at 500 cells/mm² in 384-well plates (353961, Falcon, MA, USA) for experiments with the different molecular coatings.
To validate our AI image analysis method, we selected four cultures with very different phenotypes based on conventional naked-eye microscopy observations, representative of the various endothelial qualities, ranging from the poorest (non-transplantable) to the best (the desired goal, i.e. deemed suitable for clinical application), as illustrated in Fig. 1. Culture 1 underwent EndoMT. Culture 2 was typical of a double population of normal and likely senescent cells. Culture 3 and 4 both were clinically acceptable compared to the initial clinical trial9. For this step, each of the 4 cultures was cultured on plates coated with iMatrix-511 (the reference coating) in 384 wells with 16 replicates per culture.
In a second step, we used this validated AI image analysis method to evaluate 13 coating molecules on 8 different cultures. The diversity of the parameters involved, particularly the inter-donor variability and the variability associated with the coating molecules, made it impossible to subjectively discriminate by the naked eye, which justified the use of an automated analysis method. The 8 cultures were selected based on their ECD higher than 1000 cells/mm². This rather low threshold (by reference to the usual eye bank lower threshold of 2000 cells/mm2 for transplantation) was chosen because the aim of this work was to screen for conditions likely to increase final ECD.
For the screening of molecular coating, for each coating condition tested, 8 replicates were performed for each of the 8 different corneal donors (1 coating molecule, 1 donor, 8 repetitions). We selected 9 laminin isoforms (all from BioLamina, Sundbyberg, Sweden) as well as iMatrix-511 (Nippi) and 3 types of collagens (all from Sigma Aldrich) (Table 2). Laminins differed in their chain composition, tissue localization, and biological functions, making them of interest for specific applications in regenerative medicine and tissue engineering. These 13 molecules were compared to a negative control (uncoated well). The coating with the ten laminin molecules, including the control, was performed overnight at 4 °C, as recommended by the supplier, with all at 1 µg/cm² except for iMatrix-511, which was coated at 0.5 µg/cm². For the three collagen molecules, incubation time was done at 37 °C until complete drying of the well. Collagens I and IV were performed at 6 µg/cm² and collagen II at 2 µg/cm².
Immunocytochemistry
At confluence, after approximately 3 weeks of culture, immunocytochemistry (ICC) was performed on hCECs directly in culture plates. The ICC protocol was previously described21,33. Briefly, cells were rinsed in Ca2+ and Mg2+ DPBS (SH30028.FS, Cytiva, Saint-Germain-en-Laye, France) and then fixed in pure methanol for 15 min at room temperature (RT). After three rinses, with Ca2+ and Mg2+ DPBS, cells were incubated in saturation buffer composed of 2% bovine serum albumin (A3059-100G, Sigma-Aldrich) and 2% goat serum (191356, MP Biomedicals, Irvine, CA, USA) for 30 min at 37 °C. The hCECs were incubated with the primary antibody for 1 h at 37˚C: NCAM (1:400, MAB24081, R&D Systems, Minneapolis, MN, USA). After three rinses in PBS, the highly cross-absorbed secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit IgG (A11034, Invitrogen, Carlsbad, CA, USA)), diluted to 1/800, and DAPI diluted at 2 µg/mL in blocking buffer were incubated for one hour at 37 °C under gentle agitation. After rinsing three times, Fluoromount-G mounting medium (00-4958-02, Invitrogen) was added to each well.
Images acquisition
The samples were observed with an inverted fluorescence microscope IX81 (Olympus), equipped with CellSens Dimension software V2.3 (Olympus). Images were taken at x10 and x40 magnification at the center of each well. Images were obtained using a FITC and DAPI filters, using optimized parameters for each image (intensity of light source, exposure time, contrast, resolution) and a CMOS camera with 2024 × 2048 pixels resolution (Orcas-Flash4.0 LT+, C11440-42U30, Hamamatsu Photonics K.K., Hamamatsu city, Japan).
Endothelial cell density measurement
After staining, 8 replicate images per molecular coating were taken for each donor, to measure ECD. For this, we automated the counting of each cell nucleus (DAPI-stained) with a macro using the “Stardist” plugin (https://github.com/stardist/stardist) on Fiji Software (V2.9.0/1.53t https://fiji.sc/) using x10 magnification images34,35. The software segmented each stained nucleus and calculated the ECD according to the pixel ratio. We performed a visual inspection to ensure proper nuclear recognition and corrected any possible errors.
Cellular segmentation and morphological analysis using AI and mathematical algorithms
To characterize hCECs morphology, we developed a high-performance AI-based automatic segmentation and analysis solution for cells labeled with a lateral membrane marker (here NCAM) and a nucleus marker (DAPI). For morphological analysis, 2 images were taken per molecular coating and for each donor.
Segmentation using AI
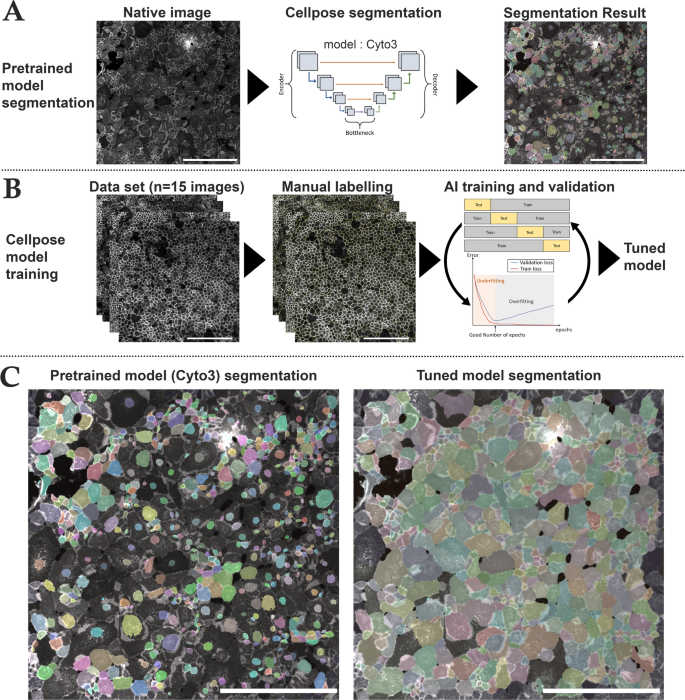
We used Cellpose, an open-source generalist deep learning algorithm for cell and nucleus segmentation (https://github.com/MouseLand/cellpose) that can be tuned for optimizing results with the users’ data (Fig. 1)36. We selected the cyto3 model because of its versatility and better efficacy than other available pretrained models37. We first verified if it was efficient with our images. We then tuned the “cyto3” Cellpose model with hCECs images labeled with NCAM and DAPI (Model in supplementary section). We manually segmented 15 images of different cell culture quality directly on the Cellpose Graphical User Interface (GUI) which allowed the extraction of the result of the segmentation on a separate image as well as the regions of interest (ROI) of each image (S13 and data in supplementary section). Manual segmentation took approximately 4–8 h per image, depending on image complexity and ECD.
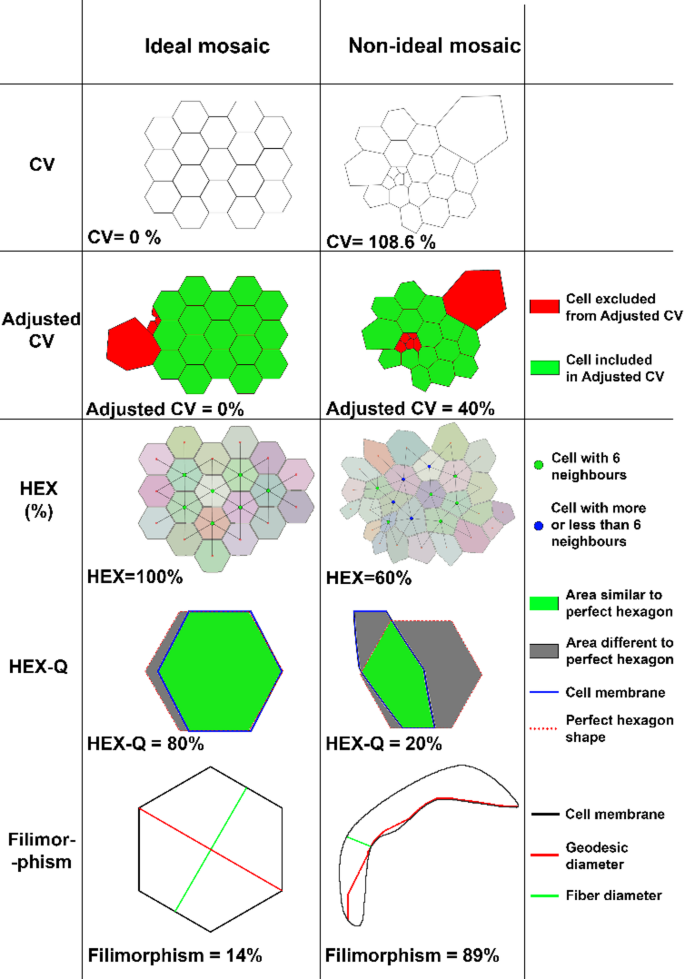
We then performed a 4-fold cross-validation on the Python version of Cellpose based on the 15 manually segmented images (2048 × 2048 pixel for 1330 × 1330 μm, 16 bits) coupled with the fluorescence images (membranes and nuclei in two different image channels). Data were divided into 60% training set, 20% test set and 20% validation set, data augmentation was performed with a factor of 4 by horizontally and vertically flipping images. As there were approximately 1500 cells per image, the training set was thus constituted of around 54,000 cells. For each fold, we optimized epochs (number of training cycles the AI went through to learn/correct the weights of the model) using the validation set (800 was the best epochs per fold). Cross-validation was done by comparing manually segmented images with Cellpose segmentation using a published dissimilarity criterion and the 6 parameters described hereafter : Endothelial Cell Density (ECD), Coefficient of variation of cell area (CV), Adjusted CV, hexagonality (HEX), quality of HEX (HEX-Q) and Filimorphism38.
Cell segmentation and AI training steps using Cellpose. (A) Steps for image segmentation with Cellpose which used an U-net model pre-trained on different cell types (Cyto3 model). The initial segmentation was not efficient on NCAM-labeled cultured corneal endothelial cells. Cell areas were coded by colors. (B) Steps for training a new model consisted in the selection of NCAM-labeled image of various quality (n = 15 images), followed by manual labeling and then a 4-fold cross validation training to optimize epochs and control segmentation quality of the newly trained model (tuned model). (C) Comparison of the same image segmented with the pre-trained Cyto3 model and with our tuned model. Scale bar = 500 μm on each image.
Morphometric analysis
We developed a custom script in Python language (V3.11.4, https://www.python.org/downloads/release/python-3114/), interpreted with Spyder IDE (V5.4.3) to measure 5 parameters on segmented images and to conduct a comprehensive analysis of cell polymegathism and pleomorphism (Fig. 2):
1/ CV of cell area: the conventional parameter for polymegathism already mentioned. The lower the value, the more homogeneous the mosaic. It was calculated by the formula \(\:{CV}_{area}=\frac{{SD}_{area}}{{Mean}_{area}}\). The CV of a healthy endothelium ranged from 26 to 35%, depending on the patient’s age39,40,41,42,43; 2/ Adjusted CV was specifically designed for this study to assess cell cultures containing several cell populations in terms of area. This adjusted CV allowed studying the CV of the majority population within the culture by replacing the mean with the median and the standard deviation with one calculated from median absolute deviation (see Supplementary methods); 3/ HEX for polymorphism. The HEX of a healthy endothelium ranged from 45 to 70% depending on the patient’s age39,40,41,42,43; 4/ Quality of hexagonality (HEX-Q) was also specifically designed for this study: among hexagonal cells (with 6 neighbors), it measured their proximity to a perfect hexagon based on the HEXADEV criterion44. HEX-Q calculation took into account the convexity of the cell as well as the length of the sides and the angle of each side of the hexagonal cell (see Supplementary methods); 5/ Filimorphism: this parameter, specifically designed for this study assessed cell elongation by modifying the aspect ratio formula (depending on definitions, aspect ratio in the literature is calculated either 1- as the ratio between the length of the major axis and the minor axis of the cell’s fitting ellipse or 2- as the ratio between the maximal and minimal Feret diameter of a cell) adapting it even for extremely concave (U-shaped) cells (see Supplementary methods).
Establishment of endothelial quality score
To comprehensively evaluate the different parameters, a score was established, hereafter referred to as the Endothelial Quality Score (EQS). This scoring system assigned 50% weight to ECD, as it is the primary criterion used in clinical practice (eye banking), and 50% to morphology, considering that both were equally important. Morphology evaluation was based on the five parameters previously described: HEX, HEX-Q, CV, adjusted CV and filimorphism, each contributing 1/10 of the EQS. The EQS was derived from Z-scores, which were calculated by the following formula: \(\:{Z}_{i}=\:\frac{{X}_{i}-\mu\:}{\sigma\:}\:\); where \(\:{Z}_{i}\) was the score value, Xi the raw value, µ the population mean for the given criterion, and σ the standard deviation of the referred population. The higher the EQS, the better the endothelial quality.
Statistical analysis
To analyze the results of the algorithm using the AI model, the GraphPad Prism software was used. Initially, the normality of each culture for each parameter was tested using three different tests (Kolmogorov-Smirnov test, Agostino and Pearson omnibus normality test, and Shapiro-Wilk normality test). If one of these three tests indicated normality for the four cultures, a repeated Measures ANOVA followed by Bonferroni’s multiple comparison test was performed as a post-hoc analysis. If none of the normality tests showed a normal distribution, a Friedman test was used, followed by Dunn’s multiple comparison post-hoc test.
To evaluate the relationship between coating molecules and ECD or morphology, a linear mixed-effect model was used. ECD values and other positive criteria were log-transformed before the mixed effect analysis. This model accounted for both fixed effects, such as factors of interest (coating molecule), and random effects (donor, replicate ID), to account for inter-individual variations and correlations between observations. Statistical analyses were performed using R software (version 4.4.1), with the “lme4” and “lmerTest” packages to fit a linear mixed-effects model. Results were expressed as the ratios of the ECD effect relative to the reference molecule with the corresponding 95% confidence intervals.
AI Research
Oxford secures £118m for AI-driven vaccine research with Ellison Institute
The University of Oxford has launched a £118m AI vaccine research programme, CoI-AI, with the Ellison Institute, aiming to combat antibiotic resistance through human challenge trials
A new initiative, led by Oxford Vaccine Group, named CoI-AI (Correlates of Immunity-Artificial Intelligence), will combine Oxford’s expertise in human challenge studies, immune science, and vaccine development with the Ellison Institute of Technology (EIT)’s Artificial Intelligence (AI) technology. The Ellison Institute will provide its advanced AI technology to the initiative, enhancing our understanding of infections and how vaccines protect us.
CoI-AI programme: Combining human challenge trials and artificial intelligence
The CoI-AI programme will focus on how the immune system responds to germs that cause serious infections and contribute to antibiotic resistance, such as Streptococcus pneumoniae, Staphylococcus aureus, and E. coli. Researchers will utilise human challenge models, where volunteers are safely exposed to bacteria under controlled conditions, and apply modern immunology and AI tools to pinpoint the immune responses that predict protection.
The CoI-AI programme, established in December 2024 through a strategic alliance between the University of Oxford and EIT, aims to develop solutions and create future leaders needed to tackle some of the most significant and challenging challenges facing humanity, thereby significantly impacting the global health landscape.
EIT is renowned for its groundbreaking research and commercial capabilities, driving scientific discoveries and fostering sustainable and ethical companies. The Ellison Institute combines cross-cutting capabilities and expertise in generative biology, clinical medicine, plant science, sustainable energy, public policy, and other related fields. The research is supported by substantial computing capability enabled by Oracle, a world-class Artificial Intelligence team, and a Scholars programme which aims to help the world’s finest up-and-coming scientists.
Tackling urgent problems in infectious diseases
Professor Sir Andrew Pollard, Director of the Oxford Vaccine Group, said: “This programme addresses one of the most urgent problems in infectious disease by helping us to understand immunity more deeply to develop innovative vaccines against deadly diseases that have so far evaded our attempts at prevention. By combining advanced immunology with artificial intelligence and utilising human challenge models to study diseases, CoI-AI will provide the tools necessary to tackle serious infections and mitigate the growing threat of antibiotic resistance. This is a new frontier in vaccine science.”
Professor Daniela Ferreira, Deputy Director of the Oxford Vaccine Group, said: “This programme will give us completely new tools to study how vaccines work at both a cellular and system-wide level, by studying infections in real time, in people, and using smart immunology tools and data to find the answers. This will open up whole new avenues to vaccine design as we improve our understanding of infection and immunity.”
Larry Ellison, Chairman of the Ellison Institute of Technology, said: “Researchers in the CoI-AI programme will use Artificial Intelligence models developed at EIT to identify and better understand the immune responses that predict protection. This vaccine development programme combines Oxford’s leadership in immunology and human challenge models with cutting-edge AI, laying the groundwork for a new era of vaccine discovery – one that is faster, smarter, and better able to respond to infectious disease outbreaks throughout the world.”
Professor Irene Tracey, Vice-Chancellor of the University of Oxford, said: “This is a major step forward in our strategic alliance with the Ellison Institute. Together, we’re combining Oxford’s strengths in vaccine science with EIT’s bold vision to tackle some of the toughest problems in global health. This is about drawing more talent and capacity to the Oxford ecosystem to turn scientific challenges into real solutions for the world.”
AI Research
National AI congress in natural resources to spotlight challenges in education, research

TEHRAN – The first national congress on artificial intelligence (AI) in natural resources aims to focus on challenges in education, research, and its applications in agriculture, natural resources, and the environment.
The two-day event will be held from September 4–5 at Shiraz University, Fars Province, with the theme ‘intelligent technology, green land, and sustainable future.
It highlights the integration of emerging technologies, such as AI, in research and technological projects.
Over 30 lecturers will share their scientific and research achievements through presentations divided into six panels and two specialized meetings, ISNA reported.
Two specialized sessions titled ‘Artificial Intelligence: Governance in Education and Research’ and ‘Precision Agriculture: Adoption, Education, Infrastructure’ will focus on AI challenges in education, research, and its application in agriculture.
The congress will be centered around productivity and promotion of natural and agricultural industries, smart management of water resources and climate, meteorology, climate change and ecosystem, sustainable agriculture, smart agriculture, big data and agricultural development, biodiversity and pests, remote sensing of natural resources, nature management, improvement of food quality and security, smart robotics, technological education, as well as ethical research in agriculture.
Some $100 million allocated to develop AI
In April, the Vice Presidency for Science, Technology, and Knowledge-Based Economy and the National Development Fund (NDF) signed a memorandum of understanding (MOU) to create a 100-million-dollar fund for the development of the artificial intelligence (AI) sector in the country.
Signed by Hossein Afshin, an official with the vice-presidency of science and technology, and Mehdi Ghazanfari, the head of the NDF, the MOU aims to establish a framework for the development and implementation of AI in line with the seventh national development plan (2023-2027) and the Leader of the Islamic Revolution Ayatollah Seyyed Ali Khamenei’s emphasis on investing in emerging fields, ISNA reported.
Accordingly, the projects introduced by the vice-presidency for science and technology will be funded through loans, partnerships, and other ways of financing. To further boost cooperation between universities and the private sector, the NDF will grant specific loans to those companies that financially support AI-based projects in universities and scientific centers.
In return, the vice-presidency for science and technology will use financing tools for emerging technologies.
MT/MG
AI Research
Accenture buys generative AI business | News
US – Accenture has agreed to acquire generative AI consulting company NeuraFlash for an undisclosed amount subject to customary closing conditions.
The deal will help strengthen Accenture’s Salesforce gen AI and managed services capabilities, with NeuraFlash focused on agentic AI tools for sales, services and field service operations.
The acquisition will see 510 staff at NeuraFlash join Accenture, primarily in North America with additional staff in Colombia and India.
Accenture said that in addition to NeuraFlash’s Salesforce and Agentforce implementation experience, the business also has Amazon Web Services capabilities, delivering personalised experiences for contact centre customers with machine learning and generative AI.
NeuraFlash is based in Massachusetts and was founded in 2016, specialising in using generative AI in complex business processes, agentic programs, analytics, in managing change and providing ongoing managed services.
Stephanie Sadowski, senior managing director and Salesforce Business Group global lead for Accenture, said: “This acquisition will significantly enhance our agentic AI capabilities and allow us to better serve the mid-market, in direct alignment with Salesforce’s strategic direction.
“By integrating NeuraFlash’s expertise, we aim to help accelerate enterprise AI adoption and drive innovation for clients across industries.”
T Brett Chisholm, chef executive and co-founder at NeuraFlash, added: “Joining forces with Accenture will help us continue to scale, amplify our impact globally and expand our gen AI capabilities to create new, exciting avenues for innovation.”
-

 Business3 days ago
Business3 days agoThe Guardian view on Trump and the Fed: independence is no substitute for accountability | Editorial
-
Tools & Platforms3 weeks ago
Building Trust in Military AI Starts with Opening the Black Box – War on the Rocks
-

 Ethics & Policy1 month ago
Ethics & Policy1 month agoSDAIA Supports Saudi Arabia’s Leadership in Shaping Global AI Ethics, Policy, and Research – وكالة الأنباء السعودية
-

 Events & Conferences3 months ago
Events & Conferences3 months agoJourney to 1000 models: Scaling Instagram’s recommendation system
-

 Jobs & Careers2 months ago
Jobs & Careers2 months agoMumbai-based Perplexity Alternative Has 60k+ Users Without Funding
-

 Funding & Business2 months ago
Funding & Business2 months agoKayak and Expedia race to build AI travel agents that turn social posts into itineraries
-

 Education2 months ago
Education2 months agoVEX Robotics launches AI-powered classroom robotics system
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoHappy 4th of July! 🎆 Made with Veo 3 in Gemini
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoOpenAI 🤝 @teamganassi
-

 Mergers & Acquisitions2 months ago
Mergers & Acquisitions2 months agoDonald Trump suggests US government review subsidies to Elon Musk’s companies