AI Research
Harnessing artificial intelligence to identify Bufalin as a molecular glue degrader of estrogen receptor alpha
Ethics approval
The experiments were approved by the Medical Ethics Review Committee of Xiangya Hospital of Central South University (Ethics code: 2023121169). Tissue samples were collected from the Xiangya Hospital of Central South University (Changsha, China), and all individuals provided informed consent prior to participating in the study.
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Central South University (CSU-2023-0462), and all procedures were conducted in accordance with the institutional guidelines of the Animal Care and Use Committee of Central South University. All mice were housed in the Laboratory Animal Research Center of Central South University, which is a pathogen-free animal facility at a controlled temperature under standard laboratory conditions (12 h light/dark cycle, temperature kept at 21–24 °C and 40–70% humidity) with food and water provided ad libitum. Female mice were selected because the study focuses on breast cancer, a disease that predominantly affects females and is influenced by female-specific hormonal and physiological factors. In compliance with ethical regulations, tumor volume did not exceed 2000 mm³, and no single tumor dimension exceeded 20 mm in diameter.
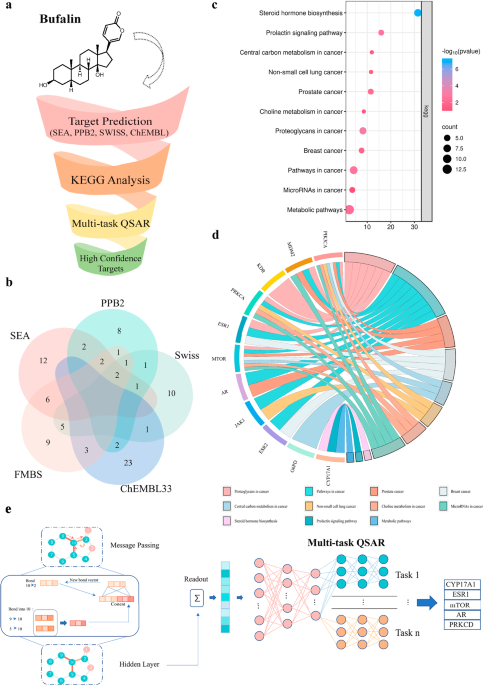
Target prediction of Bufalin based on an integrated multi-predictive strategy
To refine the target prediction range, we trained a deep learning model based on the Chemprop25 package using binding data from 11 targets sourced from ChEMBL and PubChem, with an average of 1187 compounds per target (Supplementary Table 2). The dataset was randomly split into an 80% training set and a 20% test set.
The model employs a Graph Neural Network (GNN) architecture specifically designed to learn molecular representations from graph-structured data. It consists of four primary components: (1) a shared local feature encoder that extracts atom and bond features across all tasks; (2) a directed message-passing process that propagates information along directed edges to generate atom embeddings; (3) an aggregation module that combines atom embeddings into a single molecular representation using sum or mean pooling; and (4) a feed-forward network (FFN) with task-specific multi-layer perceptron (MLP) layers to map molecular embeddings to target properties, enabling efficient multi-task predictions.
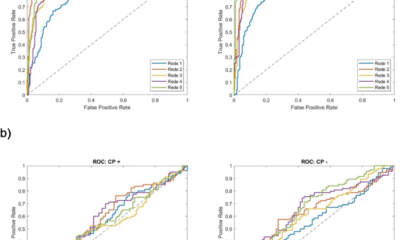
The model was trained with the Adam optimizer, incorporating learning rate scheduling, dropout regularization, and a model-ensembling strategy. Together with an advanced GNN architecture and a carefully curated dataset, this results in a robust and high-performing model, achieving a receiver operating characteristic area under the curve (ROC-AUC) of 0.94 on the test set (Supplementary Table 3). Finally, a default probability threshold of 0.8 was applied, ensuring that only targets meeting this performance criterion were selected.
Cell lines and culture
All cell lines were maintained at 37 °C in a humidified atmosphere of 5% CO2/95% air. MCF-7 cells were purchased from Cell Bank (Chinese Academy of Sciences, Beijing, China). The T47D and 293 T cell lines were purchased from Cell Bank (Chinese Academy of Sciences, Shanghai, China). The Tamoxifen-resistant cells LCC2 were derived from Wuhan University. MCF-7 and 293 T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Carlsbad, CA, USA) with 10% Fetal Bovine Serum and 1% penicillin streptomycin. T47D were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) with 10% Fetal Bovine Serum and 1% penicillin streptomycin. Tamoxifen-resistant LCC2 cells were cultured according to the literature52, briefly, LCC-2 cell lines were cultured in RPMI-1640 medium (10% Fetal Bovine Serum and 1% penicillin streptomycin). All cell lines were tested negative for mycoplasma contamination.
Reagents and Antibodies
Bufalin was purchased from APExBIO (USA). Fulvestrant, Estradiol (E2), and MG-132 were purchased from MedChemExpress (Monmouth Junction, NJ, USA). MLN4924 was purchased from TargetMol (Washington, USA). The recombinant Human CYP17A1 (cat. no. CSB-EP006392HU) and estrogen receptor (ESR1) (Cat. no. CSB-YP007830HU) proteins were purchased from CUSABIO. Recombinant human PKC delta protein (ab60844) was purchased from Abcam (Cambridge, UK). Antibodies targeting ERα (Cat. no. 8644, WB, 1:1000), LC3 (Cat. no. 12741, WB,1:1000), PARP (Cat. no. 9532, WB,1:1000), and Bcl-2 (Cat. no.15071, WB, 1:1000) were purchased from Cell Signaling Technology (Danvers, MA, USA). The ERα (cat. no. 84564-4-RR, IHC, 1:500, IF, 1:250), STUB1 (Cat. no. 68407-1-Ig, WB, 1:1000), and β-actin (Cat. no. 81115-1-RR, WB, 1:5000) antibodies were purchased from Proteintech (Chicago, IL, USA). The Flag (cat. no. M185, WB, 1:1000) and HA antibodies (cat. no. M180, WB, 1:1000) were purchased from MBL (Tokyo, Japan), while the antibody against ubiquitin (cat. no. sc-8017, WB, 1:200) was purchased from Santa Cruz Biotechnology. Anti-mouse and anti-rabbit secondary antibodies were purchased from Abiowell (Shanghai, China). Streptavidin FITC (Cat. no. 11-4317-87) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Magnetic streptavidin beads (cat. no. 22305-1) were purchased from Beaver (Suzhou, China). Lipofectamine 8000 was purchased from Beyotime Biotechnology (Shanghai, China). Lipofectamine™ RNAiMAX was purchased from Invitrogen. Protein A/G agarose beads (cat. no. 10121) were obtained from Santa Cruz Biotechnology, and the CCK-8 was purchased from Bimake (Shanghai, China). An enhanced chemiluminescence kit (cat. no. BL520A) was purchased from BioSharp (Shanghai, China).
Plasmid and siRNA transfection
Plasmids encoding wild-type and mutant ERα were obtained from Gene (Shanghai, China), while siRNAs targeting ESR1 and STUB1 were purchased from GenePharma (Suzhou, China). The target sequence of ESR1 siRNA was as follows: GCACCCUCUUGUAUUCCUATT (sense), UAGGAAUACAAGAGGGUGCTT (antisense). The target sequence of STUB1 siRNA was as follows: GCAGUCUGUGAAGGCGCACTT (sense), GUGCGCCUUCACAGACUGCTT (antisense). For siRNA transfection, the siRNA targeting ESR1 or STUB1 was incubated with Lipofectamine™ RNAiMAX in serum-free medium according to the manufacturer’s instructions. The plasmid was transfected using the Lipofectamine 8000 reagent in serum-free DMEM, according to the manufacturer’s instructions.
Western Blot
After treatment, the cells were washed twice with cold PBS and lysed on ice for 30 minutes in RIPA lysis (Abiowell) supplemented with a protease inhibitor cocktail (Biotool), followed by centrifugation at 12,000 g for 15 minutes at 4 °C. The protein concentration of the supernatant was determined using BCA. Proteins were resolved by SDS-PAGE and then transferred to PVDF membrane (Merck KGaA, Darmstadt, Germany). The PVDF membranes were blocked with 5% skim milk for 1 h at room temperature and then incubated with the respective antibodies at 4 °C for 14 h. After 14 h the PVDF membranes were washed thrice with PBST and incubated with a secondary antibody at room temperature for 1 h. The signals were detected by chemiluminescence assay using a ChemiDoc Touch (Bio-Rad).
Cell viability assays
Briefly, cells were seeded into 96-well plates at an appropriate density and allowed to adhere overnight. The following day, cells were treated with various concentrations of the indicated drug and incubated for the desired time period. Subsequently, 10 μL of CCK-8 solution was added to each well and the plates were incubated at 37 °C for 1-2 hours. After incubation, absorbance was measured at 450 nm using a microplate reader.
Colony forming assay
MCF-7, T47D, and LCC2 cells were seeded in 6-well plates, exposed to the indicated treatments, and cultured for approximately 15 days, and the medium was changed every 3 days. After treatment, the cells were fixed with 4% paraformaldehyde, stained with crystal violet for 24 h, washed with water, and colonies were counted.
5-Ethynyl-2’- deoxyuridine Assay (EdU)
After treatment with Bufalin, LCC2 cells were incubated with 5-ethynyl-2′-deoxyuridine (EdU; RiboBio, Guangzhou, China) for 2 hours at 37 °C, according to the manufacturer’s instructions. The cells were fixed with 4% paraformaldehyde at room temperature for 30 min, followed by treatment with 2 mg/mL glycine for 5 min. Next, the cells were permeabilized with 0.5% Triton X-100 for 10 min and stained with a 1× Apollo reaction cocktail for 30 min in the dark at room temperature. Finally, cell nuclei were counterstained with Hoechst 33342 for 30 min at room temperature. Images were captured using a fluorescence microscope.
Quantitative Real-time PCR
Total RNA was isolated from cells using the TRIzol reagent (CWBio, Taizhou, China), and reverse transcription was carried out using the PrimeScript RT reagent kit (TaKaRa, Japan) to generate complementary DNA (cDNA). Quantitative real-time PCR was conducted on a QuantStudio Real-Time PCR System (Life Technologies) using QuantStudio Design & Analysis Software v1.5.1. Gene expression levels were quantified using the standard 2−ΔΔCt method. The qPCR primer sets were: ESR1: GGGAAGTATGGCTATGGAATCTG (forward), TGGCTGGACACATATAGTCGTT (reverse); AGR2: AGAGCAGTTTGTCCTCCTCAA (forward), CAGGTTCGTAAGCATAGAGACG (reverse); CCND1: CAATGACCCCGCACGATTTC (forward), CATGGAGGGCGGATTGGAA (reverse); GREB1: TGGTCCGTAATGCACAAGGG (forward), CTGCGTTTAGTGAGGGGTGA (reverse); NRIP1: GGATCAGGTACTGCCGTTGAC (forward), CTGGACCATTACTTTGACAGGTG (reverse); PGR: TTATGGTGTCCTTACCTGTGGG (forward), GCGGATTTTATCAACGATGCAG (reverse); SIAH2: TCTTCGAGTGTCCGGTCTG (forward), CGGCATTGGTTACACACCAG (reverse), GAPDH: TGACATCAAGAAGGTGGTGAAGCAG (forward), GTGTCGCTGTTGAAGTCAGAGGAG (reverse).
Flow-cytometric analysis of apoptosis
After Bufalin treatment, the cells were collected and washed twice with cold PBS. Subsequently, 5 μL Annexin V-FITC and 5 μL propidium iodide (PI) staining buffer were added to the cells and incubated in the dark at room temperature. After 15 min, the stained cells were analyzed using FACS.
Immunofluorescence staining
MCF-7 cells treated with Biotin-Bufalin on glass coverslips were fixed in 4% paraformaldehyde for 30 min at room temperature and blocked with 5% bovine serum albumin (BSA) for 1 h. The fixed cells were then incubated with anti-ERα antibody and streptavidin FITC at 4 °C overnight, followed by Alexa Fluor 594 anti-rabbit IgG antibody. At the end of the incubation period, the cells’ nuclei were stained with DAPI, and the fluorescence signal was detected and captured using confocal microscopy.
Co-immunoprecipitation (Co-IP) assay
The 293 T cells were transiently transfected with Flag-ERα plasmid or HA-STUB1 and subjected to Bufalin treatment as indicated. After treatment, the cells were washed twice with PBS and lysed in mammalian protein extraction reagent buffer (cat. no. 78501; Thermo Scientific), supplemented with protease and phosphatase inhibitors for 30 min. Cell lysates were centrifuged, and supernatant was precleared with protein G agarose beads (Santa Cruz), then subjected to immunoprecipitation with indicated antibodies and protein A/G agarose beads at 4 °C overnight. The next day, the immunocomplexes were washed five times with PBS, and the binding proteins were eluted by 1 × SDS-PAGE loading buffer at 95 °C for 10 min. Bound proteins were identified using immunoblotting and western blot.
Biotin-pull down assay
After treatment, the cells were washed with PBS and lysed for 30 min in mammalian protein extraction (Thermo Scientific) with protease and phosphatase inhibitors. Following cell lysis, the supernatants were collected by centrifugation at 12,000 × g for 15 minutes at 4 °C. The streptavidin magnetic beads were pre-incubated with the cell lysate for 2 hours. Subsequently, 500 μg of clarified cell lysate was incubated with either D-Biotin or Biotin-Bufalin overnight at 4 °C for target protein capture. The following day, the mixture containing biotin and cell lysate was coupled with streptavidin magnetic beads (Beaver) for 2 hours at room temperature. The magnetic beads were then collected using a magnetic rack and washed six times to remove non-specifically bound proteins. Bound proteins were eluted by boiling in 1× SDS-PAGE loading buffer at 95 °C for 10 minutes, followed by detection via western blot analysis.
Animal studies
Briefly, the Tamoxifen-resistant cells LCC2 (2 × 106 cells) were subcutaneously injected into 4-week-old female nude mice (Hunan Slack Jingda Laboratory Animal Co., Ltd.). Tumor sizes were measured on different days after inoculation and calculated using the formula V = lw2π/6, where l is the length and w is the width. When the tumors were palpable, the mice were randomly divided into designated groups and received the indicated treatments. The tumor volume was measured using a Vernier caliper every two days.
Immunohistochemistry (IHC)
The human cancer tissue specimens from patients with Tamoxifen treatment and recurrence, and animal tissue were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. After deparaffinization, antigen retrieval was performed using a citric acid buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and following blocked with the application of normal serum. Sections were then incubated overnight at 4 °C with the antibodies against ERα and Ki67, followed by HRP-conjugated secondary antibodies. The signal was developed using DAB, and the nuclei were counterstained with hematoxylin. Immunohistochemical staining was performed according to the manufacturer’s protocol.
Protein-ligand binding conformation modeling
The conformation of the protein-ligand complex was modeled using Schrödinger software (version 2022.1). The structure of Bufalin was obtained from PubChem, and ligand conformations were generated using LigPrep. The crystal structure of the ESR1 protein was obtained from the Protein Data Bank (PDB ID: 3ERT)63. The Glide SP protocol was employed to generate the optimal conformation of the protein-ligand complex.
Protein-protein docking conformation modeling
The structures of STUB1, TRIM56, RNF181, RNF2, and ESR1 proteins were obtained from the Protein Data Bank and Alphafold 3, and subjected to 15 repeats of optimization using the relax module of Rosetta (version 3.5.1)64. Ten conformations were generated from each optimization, and the top-scoring conformation from Rosetta was used for subsequent protein-protein docking. Initial protein-protein docking was performed using the ClusPro server65. Complex conformations with incorrect STUB1, TRIM56, RNF181, and RNF2 binding modes were excluded from the ClusPro docking results. These conformations were subjected to structural refinement using the docking protocol of Rosetta. All generated conformations were ranked according to their Rosetta scores.
Molecular dynamic simulations
Molecular simulations of ESR1-Bufalin, ESR1-STUB1, and ESR1-Bufalin-STUB1 complexes were conducted using Amber22 software. Proteins and ligands were parameterized using Amber ff19SB66 and GAFF267 force fields, respectively. The complex systems were solvated in a TIP3P water box extending 10 Å from the protein. Chloride and sodium ions were then added to neutralize the system. Subsequently, energy minimization was performed for up to 20,000 steps. The systems were heated from 0 K to 298.15 K over 100 ps and the pressure was increased to atmospheric pressure over another 100 ps, with a time step of 1 fs during the heating and pressurization processes. Finally, a 200 ns production classical MD simulation was conducted with a time step of 2 fs. Trajectory analysis was performed using CPPTRAJ.
MM-PBSA and alanine scanning mutations analysis
MM-PBSA binding free energy calculations were performed using MMPBSA.py from AmberTools202368, using only the last 100 ns of the trajectory. We extracted 100 frames from the trajectory for subsequent energy calculations. In addition, residual energy decomposition was performed for the Bufalin-ESR1 complex. Alanine scanning mutations69 were conducted on the top ten residues from the energy decomposition analysis.
Surface Plasmon Resonance (SPR) assay
After storage at -80 °C, Bufalin and the recombinant proteins CYP17A1, ESR1, and PKC delta were allowed to equilibrate to room temperature. Bufalin was diluted with DMSO to the appropriate concentration for spotting and used as the immobilized phase. The working solution was printed onto a 3D photocrosslinkable sensor chip using the BioDot™ AD1520 microarray printer, with four nonadjacent replicate spots per compound. The printed chips were vacuum-dried and then subjected to UV-induced photocrosslinking using a crosslinking instrument. Following crosslinking, the chips were sequentially washed on a shaker with DMF, ethanol (EtOH), and deionized water for 15 minutes each. The recombinant protein samples were diluted to generate five concentration gradients: 200 nM, 400 nM, 800 nM, 1600 nM, and 3200 nM, and were injected over the chip surface. During interaction analysis, the protein analytes were passed over the chip surface at a flow rate of 0.5 μL/s. Each association phase lasted 600 seconds, followed by a 360-second dissociation phase. After each binding cycle, the chip surface was regenerated with 10 mM glycine-HCl (pH 2.0) at a flow rate of 2 μL/s to remove the bound analytes.
Patient-derived organoid
Tissue specimens from patients who relapsed after Tamoxifen therapy were processed for organoid culture according to previous protocols70,71. Samples were obtained from the Xiangya Hospital of Central South University (Changsha, China), and written informed consent was obtained from all participants prior to collection. Tissue samples were enzymatically digested into single-cell suspensions using collagenase (Sigma) and subsequently cultured in a 3D environment composed of 50% chilled Matrigel (Corning). After 5 days of culture, the resulting organoids were dissociated into single-cell suspensions, seeded into 384-well plates, and treated with Bufalin. After 4 days of treatment, cell viability was assessed using CellTiter-Glo 3D Reagent (Promega) according to the manufacturer’s instructions.
Organoid live/dead cell staining
Live/dead organoid cell staining was performed as previously described72. Following treatment, the samples were washed twice with cold PBS and subsequently stained with Calcein-AM and propidium iodide (PI) at 37 °C for 20 minutes in the dark. Finally, the images were captured using a fluorescence microscope.
Statistics and reproducibility
We analyzed the data using GraphPad Prism 9.0 software. All samples represent biological replicates, and the statistical measurements are presented as mean ± SD as specified in each figure. No statistical method was used to predetermine sample size. No data were excluded from the analyses. The experiments were randomized. The Investigators were blinded to allocation during experiments and outcome assessment. Statistical analyses were performed using one-way or two-way ANOVA, depending on the experimental design. A P-value < 0.05 was considered statistically significant. The P values are given in the figures. All representative experiments are repeated at least three times independently with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
AI Research
Keeping AI-generated content authentic – LAS News

Where some see artificial intelligence (AI) flattening human creativity, Kaili Meyer (‘17 journalism and mass communication) sees an opportunity to prove that authenticity and individuality are still the heart of communication.
As founder of the sales and web copywriting company Reveal Studio Co., Meyer built her business and later included AI as an extension of her work. She developed tools that train AI to preserve the integrity of an individual’s tone.
Wellness to print
Meyer began her undergraduate studies at Iowa State in kinesiology but realized she had a talent for writing.
“I thought about what I was really good at, and the answer was writing,” Meyer said.
She pivoted to a journalism and mass communication major, with a minor in psychology. Her passion for writing led her to work on student publications in the Greenlee School of Journalism and Communication, where she founded a health and wellness magazine.
That drive to build something from scratch set the tone for her entrepreneurial approach to her business.
Building an AI copywriting company
After graduation, Meyer joined Principal Financial in institutional investing, where she translated complex economic reports into accessible updates for stakeholders. She gained business skills – but her creative energy was missing.
By freelancing on the side for content, copy and magazines, she eventually replaced her salary, left corporate life, and began the process of launching her own company.
Reveal Studio Co started out with direct client interactions, grew to include a template shop, and now includes AI tools.
In 2023, the AI chatbot ChatGPT had its one-year anniversary with over 1.7 billion users. As generative AI went mainstream and pushed into more areas, Meyer was skeptical of the rapidly growing adoption of AI in society. She began to flag AI-written content everywhere and set out to prove that it could never replicate the human voice.
“In doing so, I proved myself wrong,” Meyer said.
As Meyer researched AI, she realized it could be tailored to one’s own persona.
She developed The Complete AI Copy Buddy, a training manual that teaches an AI platform to mimic an individual’s style. By completing a template and submitting it to an AI source, users can acquire anything they need – from content ideas to full pieces such as social posts, emails, web copy and business collateral – all specifically tailored to their audience, brand, and voice.
The launch of the training manual earned $60,000 in two weeks, more than her first year’s corporate salary.
That success propelled Meyer into creating The Sales & Copy Bestie, a custom Generative Pre-trained Transformer (GPT) built from her knowledge in psychology and copywriting. Contractors support her work while she keeps the control and direction.
“If people are going to use AI – which they are – I might as well help them do it better,” she said.
Meyer prioritizes sales psychology, understanding how neuroscience drives decisions and taking that information to form effective and persuasive messages.
“Copy is messaging intended to get somebody to take action,” Meyer explained. “If I don’t understand what makes someone’s brain want to take action, then I can’t write really good copy.”
Meyer’s clients range from educators and creative service providers to lawyers, accountants, and business owners seeking sharper websites, sales pages, or email funnels.
Meyers’ vision of success
Meyer attributes her growth to persistence and a pure mindset.
“I don’t view anything as failure. Everything is just a step closer to where you want to be,” she said.
This year, Meyer plans to balance her entrepreneurial success with her creative side. She is finishing a poetry book, sketching artwork, and outlining her first novel.
“I’ve spent eight years building a really successful business,” Meyer said. “Now I want to build a life outside of work that fulfills me.”
AI Research
Accelerating HPC and AI research in universities with Amazon SageMaker HyperPod
This post was written with Mohamed Hossam of Brightskies.
Research universities engaged in large-scale AI and high-performance computing (HPC) often face significant infrastructure challenges that impede innovation and delay research outcomes. Traditional on-premises HPC clusters come with long GPU procurement cycles, rigid scaling limits, and complex maintenance requirements. These obstacles restrict researchers’ ability to iterate quickly on AI workloads such as natural language processing (NLP), computer vision, and foundation model (FM) training. Amazon SageMaker HyperPod alleviates the undifferentiated heavy lifting involved in building AI models. It helps quickly scale model development tasks such as training, fine-tuning, or inference across a cluster of hundreds or thousands of AI accelerators (NVIDIA GPUs H100, A100, and others) integrated with preconfigured HPC tools and automated scaling.
In this post, we demonstrate how a research university implemented SageMaker HyperPod to accelerate AI research by using dynamic SLURM partitions, fine-grained GPU resource management, budget-aware compute cost tracking, and multi-login node load balancing—all integrated seamlessly into the SageMaker HyperPod environment.
Solution overview
Amazon SageMaker HyperPod is designed to support large-scale machine learning operations for researchers and ML scientists. The service is fully managed by AWS, removing operational overhead while maintaining enterprise-grade security and performance.
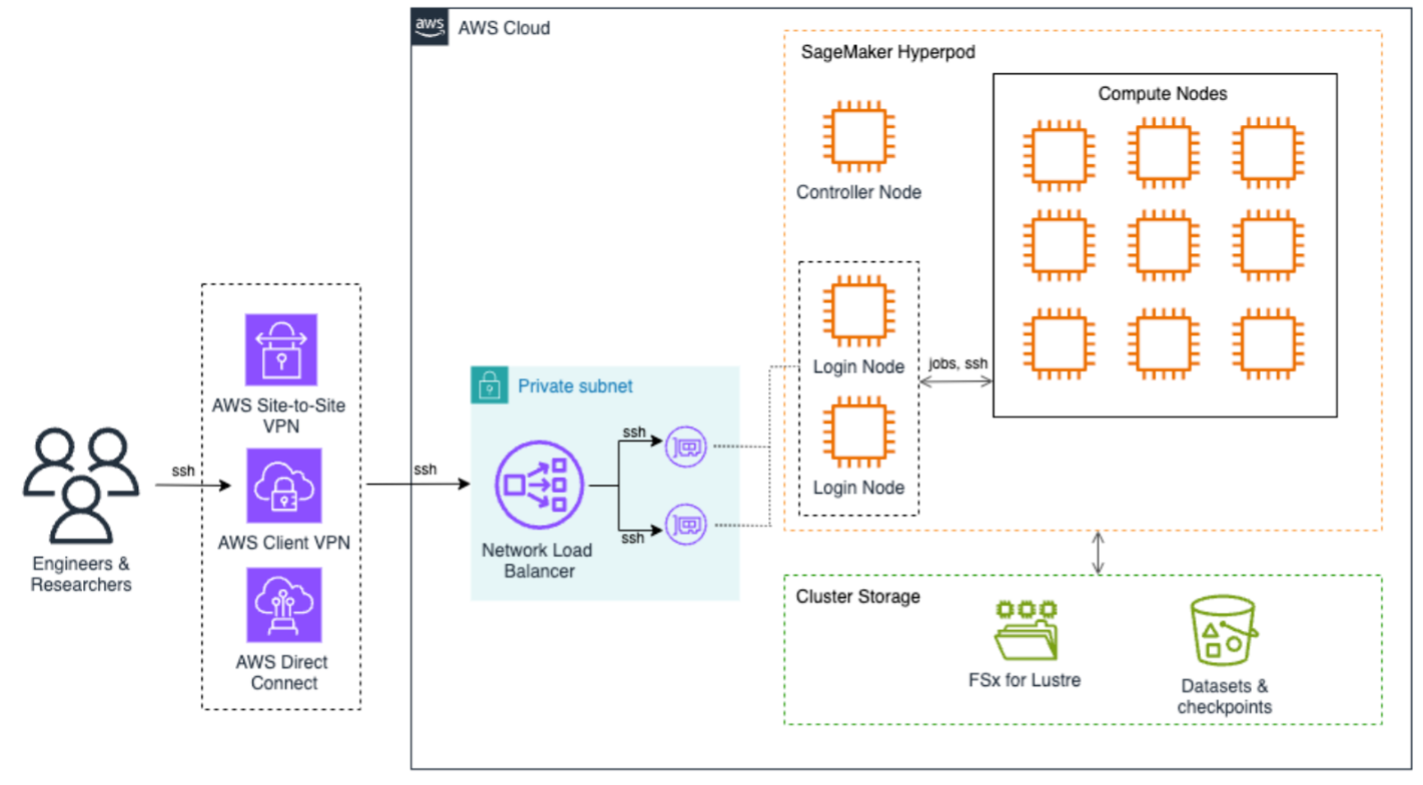
The following architecture diagram illustrates how to access SageMaker HyperPod to submit jobs. End users can use AWS Site-to-Site VPN, AWS Client VPN, or AWS Direct Connect to securely access the SageMaker HyperPod cluster. These connections terminate on the Network Load Balancer that efficiently distributes SSH traffic to login nodes, which are the primary entry points for job submission and cluster interaction. At the core of the architecture is SageMaker HyperPod compute, a controller node that orchestrates cluster operations, and multiple compute nodes arranged in a grid configuration. This setup supports efficient distributed training workloads with high-speed interconnects between nodes, all contained within a private subnet for enhanced security.
The storage infrastructure is built around two main components: Amazon FSx for Lustre provides high-performance file system capabilities, and Amazon S3 for dedicated storage for datasets and checkpoints. This dual-storage approach provides both fast data access for training workloads and secure persistence of valuable training artifacts.
The implementation consisted of several stages. In the following steps, we demonstrate how to deploy and configure the solution.
Prerequisites
Before deploying Amazon SageMaker HyperPod, make sure the following prerequisites are in place:
- AWS configuration:
- The AWS Command Line Interface (AWS CLI) configured with appropriate permissions
- Cluster configuration files prepared:
cluster-config.jsonandprovisioning-parameters.json
- Network setup:
- An AWS Identity and Management (IAM) role with permissions for the following:
Launch the CloudFormation stack
We launched an AWS CloudFormation stack to provision the necessary infrastructure components, including a VPC and subnet, FSx for Lustre file system, S3 bucket for lifecycle scripts and training data, and IAM roles with scoped permissions for cluster operation. Refer to the Amazon SageMaker HyperPod workshop for CloudFormation templates and automation scripts.
Customize SLURM cluster configuration
To align compute resources with departmental research needs, we created SLURM partitions to reflect the organizational structure, for example NLP, computer vision, and deep learning teams. We used the SLURM partition configuration to define slurm.conf with custom partitions. SLURM accounting was enabled by configuring slurmdbd and linking usage to departmental accounts and supervisors.
To support fractional GPU sharing and efficient utilization, we enabled Generic Resource (GRES) configuration. With GPU stripping, multiple users can access GPUs on the same node without contention. The GRES setup followed the guidelines from the Amazon SageMaker HyperPod workshop.
Provision and validate the cluster
We validated the cluster-config.json and provisioning-parameters.json files using the AWS CLI and a SageMaker HyperPod validation script:
Then we created the cluster:
Implement cost tracking and budget enforcement
To monitor usage and control costs, each SageMaker HyperPod resource (for example, Amazon EC2, FSx for Lustre, and others) was tagged with a unique ClusterName tag. AWS Budgets and AWS Cost Explorer reports were configured to track monthly spending per cluster. Additionally, alerts were set up to notify researchers if they approached their quota or budget thresholds.
This integration helped facilitate efficient utilization and predictable research spending.
Enable load balancing for login nodes
As the number of concurrent users increased, the university adopted a multi-login node architecture. Two login nodes were deployed in EC2 Auto Scaling groups. A Network Load Balancer was configured with target groups to route SSH and Systems Manager traffic. Lastly, AWS Lambda functions enforced session limits per user using Run-As tags with Session Manager, a capability of Systems Manager.
For details about the full implementation, see Implementing login node load balancing in SageMaker HyperPod for enhanced multi-user experience.
Configure federated access and user mapping
To facilitate secure and seamless access for researchers, the institution integrated AWS IAM Identity Center with their on-premises Active Directory (AD) using AWS Directory Service. This allowed for unified control and administration of user identities and access privileges across SageMaker HyperPod accounts. The implementation consisted of the following key components:
- Federated user integration – We mapped AD users to POSIX user names using Session Manager
run-astags, allowing fine-grained control over compute node access - Secure session management – We configured Systems Manager to make sure users access compute nodes using their own accounts, not the default
ssm-user - Identity-based tagging – Federated user names were automatically mapped to user directories, workloads, and budgets through resource tags
For full step-by-step guidance, refer to the Amazon SageMaker HyperPod workshop.
This approach streamlined user provisioning and access control while maintaining strong alignment with institutional policies and compliance requirements.
Post-deployment optimizations
To help prevent unnecessary consumption of compute resources by idle sessions, the university configured SLURM with Pluggable Authentication Modules (PAM). This setup enforces automatic logout for users after their SLURM jobs are complete or canceled, supporting prompt availability of compute nodes for queued jobs.
The configuration improved job scheduling throughput by freeing idle nodes immediately and reduced administrative overhead in managing inactive sessions.
Additionally, QoS policies were configured to control resource consumption, limit job durations, and enforce fair GPU access across users and departments. For example:
- MaxTRESPerUser – Makes sure GPU or CPU usage per user stays within defined limits
- MaxWallDurationPerJob – Helps prevent excessively long jobs from monopolizing nodes
- Priority weights – Aligns priority scheduling based on research group or project
These enhancements facilitated an optimized, balanced HPC environment that aligns with the shared infrastructure model of academic research institutions.
Clean up
To delete the resources and avoid incurring ongoing charges, complete the following steps:
- Delete the SageMaker HyperPod cluster:
- Delete the CloudFormation stack used for the SageMaker HyperPod infrastructure:
This will automatically remove associated resources, such as the VPC and subnets, FSx for Lustre file system, S3 bucket, and IAM roles. If you created these resources outside of CloudFormation, you must delete them manually.
Conclusion
SageMaker HyperPod provides research universities with a powerful, fully managed HPC solution tailored for the unique demands of AI workloads. By automating infrastructure provisioning, scaling, and resource optimization, institutions can accelerate innovation while maintaining budget control and operational efficiency. Through customized SLURM configurations, GPU sharing using GRES, federated access, and robust login node balancing, this solution highlights the potential of SageMaker HyperPod to transform research computing, so researchers can focus on science, not infrastructure.
For more details on making the most of SageMaker HyperPod, check out the SageMaker HyperPod workshop and explore further blog posts about SageMaker HyperPod.
About the authors
 Tasneem Fathima is Senior Solutions Architect at AWS. She supports Higher Education and Research customers in the United Arab Emirates to adopt cloud technologies, improve their time to science, and innovate on AWS.
Tasneem Fathima is Senior Solutions Architect at AWS. She supports Higher Education and Research customers in the United Arab Emirates to adopt cloud technologies, improve their time to science, and innovate on AWS.
 Mohamed Hossam is a Senior HPC Cloud Solutions Architect at Brightskies, specializing in high-performance computing (HPC) and AI infrastructure on AWS. He supports universities and research institutions across the Gulf and Middle East in harnessing GPU clusters, accelerating AI adoption, and migrating HPC/AI/ML workloads to the AWS Cloud. In his free time, Mohamed enjoys playing video games.
Mohamed Hossam is a Senior HPC Cloud Solutions Architect at Brightskies, specializing in high-performance computing (HPC) and AI infrastructure on AWS. He supports universities and research institutions across the Gulf and Middle East in harnessing GPU clusters, accelerating AI adoption, and migrating HPC/AI/ML workloads to the AWS Cloud. In his free time, Mohamed enjoys playing video games.
AI Research
Exploring the Real-Time Race Track with Amazon Nova
This post is co-written by Jake Friedman, President + Co-founder of Wildlife.
Amazon Nova is enhancing sports fan engagement through an immersive Formula 1 (F1)-inspired experience that turns traditional spectators into active participants. This post explores the Real-Time Race Track (RTRT), an interactive experience built using Amazon Nova in Amazon Bedrock, that lets fans design, customize, and share their own racing circuits. We highlight how generative AI capabilities come together to deliver strategic racing insights such as pit timing and tire choices, and interactive features like an AI voice assistant and a retro-style racing poster.
Evolving fan expectations and the technical barriers to real-time, multimodal engagement
Today’s sports audiences expect more than passive viewing—they want to participate, customize, and share. As fan expectations evolve, delivering engaging and interactive experiences has become essential to keeping audiences invested. Static digital content no longer holds attention; fans are drawn to immersive formats that make it possible to influence or co-create aspects of the event. For brands and rights holders, this shift presents both an opportunity and a challenge: how to deliver dynamic, meaningful engagement at scale. Delivering this level of interactivity comes with a unique set of technical challenges. It requires support for multiple modalities—text, speech, image, and data—working together in real time to create a seamless and immersive experience. Because fan-facing experiences are often offered for free, cost-efficiency becomes critical to sustain engagement at scale. And with users expecting instant responses, maintaining low-latency performance across interactions is essential to avoid disrupting the experience.
Creating immersive fan engagement with the RTRT using Amazon Nova
To foster an engaging and immersive experience, we developed the Real-Time Race Track, allowing F1 fans to design their own custom racing circuit using Amazon Nova. You can draw your track in different lengths and shapes while receiving real-time AI recommendations to modify your racing conditions. You can choose any location around the world for your race track and Amazon Nova Pro will use it to generate your track’s name and simulate realistic track conditions using that region’s weather and climate data. When your track is complete, Amazon Nova Pro analyzes the track to produce metrics like top speed and projected lap time, and offers two viable race strategies focused on tire management. You can also consult with Amazon Nova Sonic, a speech-to-speech model, for strategic track design recommendations. The experience culminates with Amazon Nova Canvas generating a retro-inspired racing poster of your custom track design that you can share or download. The following screenshots show some examples of the RTRT interface.
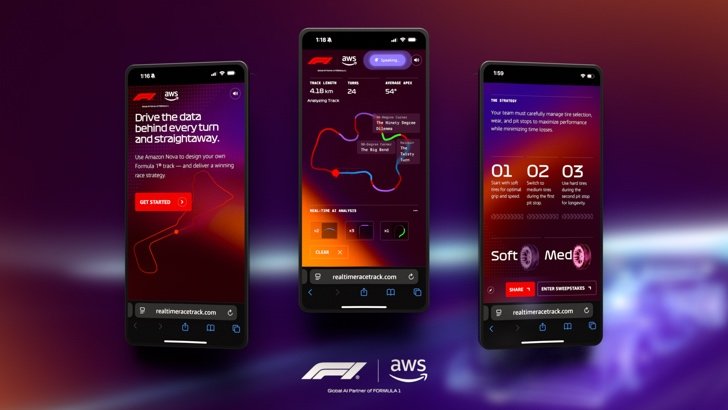 |
Amazon Nova models are cost-effective and deliver among the best price-performance in their respective class, helping enterprises create scalable fan experiences while managing costs effectively. With fast speech processing and high efficiency, Amazon Nova provides seamless, real-time, multimodal interactions that meet the demands of interactive fan engagement. Additionally, Amazon Nova comes with built-in controls to maintain the safe and responsible use of AI. Combining comprehensive capabilities, cost-effectiveness, low latency, and trusted reliability, Amazon Nova is the ideal solution for applications requiring real-time, dynamic engagement.
Prompts, inputs, and system design behind the RTRT experience
The RTRT uses the multimodal capabilities of Amazon Nova Pro to effectively lead users from a single line path drawing to a fully viable race track design, including strategic racing recommendations and a bold visual representation of their circuit in the style of a retro racing poster.
The following diagram gives an overview of the system architecture.
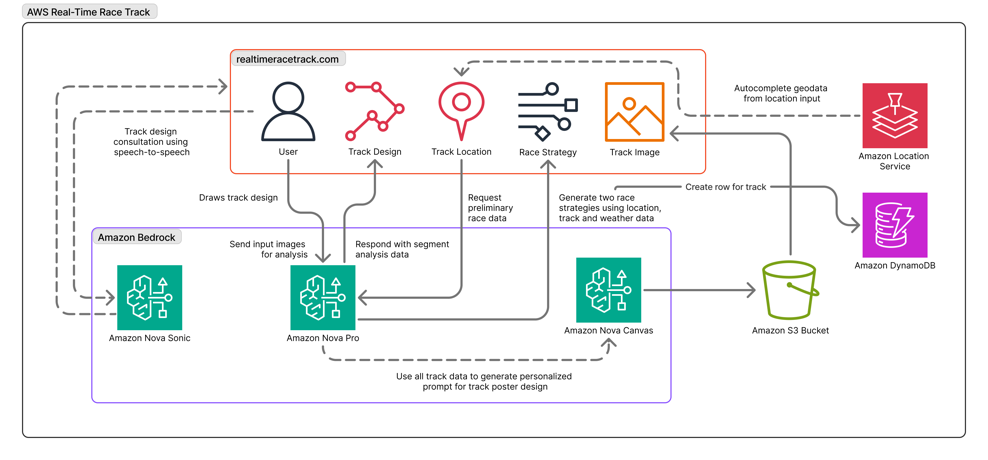
Prompt engineering plays a crucial role in delivering structured output that can flow seamlessly into the UI, which has been optimized for at-a-glance takeaways that use Amazon Nova Pro to quickly analyze multiple data inputs to accelerate users’ decision making. In the RTRT, this extends to the input images provided to Amazon Nova Pro for vision analysis. Each time the user adds new segments to their racing circuit, a version of the path is relayed to Amazon Nova Pro with visible coordinate markers that produce accurate path analysis (see the following screenshot) and corresponding output data, which can be visually represented back to users with color-coded track sectors.
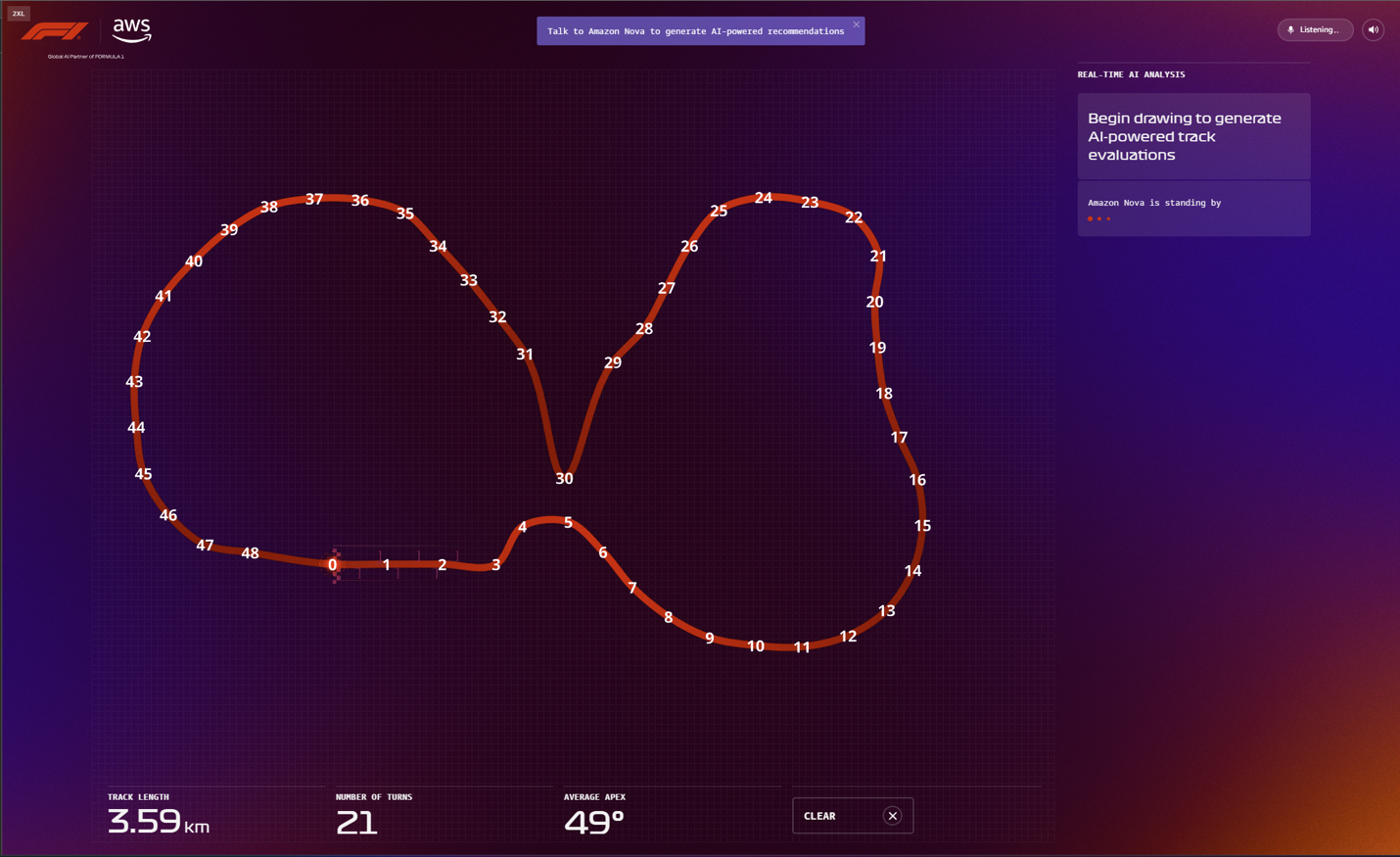
This is paired with multiple system prompts to define the role of Amazon Nova Pro at each stage of the app, as well as to return responses that are ready to be consumed by the front end.
The following is a prompt example:
The prompts also use sets of examples to produce consistent results across a diverse range of possible track designs and locations:
This is also a key stage in which to employ responsible use of AI, instructing the model not to generate content that might infringe on existing race tracks or other copyrighted material.
These considerations are essential when working with creative models like Amazon Nova Canvas. Race cars commonly feature liveries that contain a dozen or more sponsor logos. To avoid concern, and to provide the cleanest, most aesthetically appealing retro racing poster designs, Amazon Nova Canvas was given a range of conditioning images that facilitate vehicle accuracy and consistency. The images work in tandem with our prompt for a bold illustration style featuring cinematic angles.
The following is a prompt example:
The following images show the output.
 |
 |
Conclusion
The Real-Time Race Track showcases how generative AI can deliver personalized, interactive moments that resonate with modern sports audiences. Amazon Nova models power each layer of the experience, from speech and image generation to strategy and analysis, delivering rich, low-latency interactions at scale. This collaboration highlights how brands can use Amazon Nova to build tailored and engaging experiences.
About the authors
 Raechel Frick is a Sr. Product Marketing Manager at AWS. With over 20 years of experience in the tech industry, she brings a customer-first approach and growth mindset to building integrated marketing programs.
Raechel Frick is a Sr. Product Marketing Manager at AWS. With over 20 years of experience in the tech industry, she brings a customer-first approach and growth mindset to building integrated marketing programs.
 Anuj Jauhari is a Sr. Product Marketing Manager at AWS, enabling customers to innovate and achieve business impact with generative AI solutions built on Amazon Nova models.
Anuj Jauhari is a Sr. Product Marketing Manager at AWS, enabling customers to innovate and achieve business impact with generative AI solutions built on Amazon Nova models.
 Jake Friedman is the President and Co-founder at Wildlife, where he leads a team launching interactive experiences and content campaigns for global brands. His work has been recognized with the Titanium Grand Prix at the Cannes Lions International Festival of Creativity for “boundary-busting, envy-inspiring work that marks a new direction for the industry and moves it forward.”
Jake Friedman is the President and Co-founder at Wildlife, where he leads a team launching interactive experiences and content campaigns for global brands. His work has been recognized with the Titanium Grand Prix at the Cannes Lions International Festival of Creativity for “boundary-busting, envy-inspiring work that marks a new direction for the industry and moves it forward.”
-

 Business1 week ago
Business1 week agoThe Guardian view on Trump and the Fed: independence is no substitute for accountability | Editorial
-
Tools & Platforms3 weeks ago
Building Trust in Military AI Starts with Opening the Black Box – War on the Rocks
-

 Ethics & Policy1 month ago
Ethics & Policy1 month agoSDAIA Supports Saudi Arabia’s Leadership in Shaping Global AI Ethics, Policy, and Research – وكالة الأنباء السعودية
-

 Events & Conferences4 months ago
Events & Conferences4 months agoJourney to 1000 models: Scaling Instagram’s recommendation system
-

 Jobs & Careers2 months ago
Jobs & Careers2 months agoMumbai-based Perplexity Alternative Has 60k+ Users Without Funding
-

 Education2 months ago
Education2 months agoVEX Robotics launches AI-powered classroom robotics system
-

 Funding & Business2 months ago
Funding & Business2 months agoKayak and Expedia race to build AI travel agents that turn social posts into itineraries
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoHappy 4th of July! 🎆 Made with Veo 3 in Gemini
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoOpenAI 🤝 @teamganassi
-

 Education2 months ago
Education2 months agoMacron says UK and France have duty to tackle illegal migration ‘with humanity, solidarity and firmness’ – UK politics live | Politics