AI Research
Harnessing artificial intelligence to identify Bufalin as a molecular glue degrader of estrogen receptor alpha
Ethics approval
The experiments were approved by the Medical Ethics Review Committee of Xiangya Hospital of Central South University (Ethics code: 2023121169). Tissue samples were collected from the Xiangya Hospital of Central South University (Changsha, China), and all individuals provided informed consent prior to participating in the study.
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Central South University (CSU-2023-0462), and all procedures were conducted in accordance with the institutional guidelines of the Animal Care and Use Committee of Central South University. All mice were housed in the Laboratory Animal Research Center of Central South University, which is a pathogen-free animal facility at a controlled temperature under standard laboratory conditions (12 h light/dark cycle, temperature kept at 21–24 °C and 40–70% humidity) with food and water provided ad libitum. Female mice were selected because the study focuses on breast cancer, a disease that predominantly affects females and is influenced by female-specific hormonal and physiological factors. In compliance with ethical regulations, tumor volume did not exceed 2000 mm³, and no single tumor dimension exceeded 20 mm in diameter.
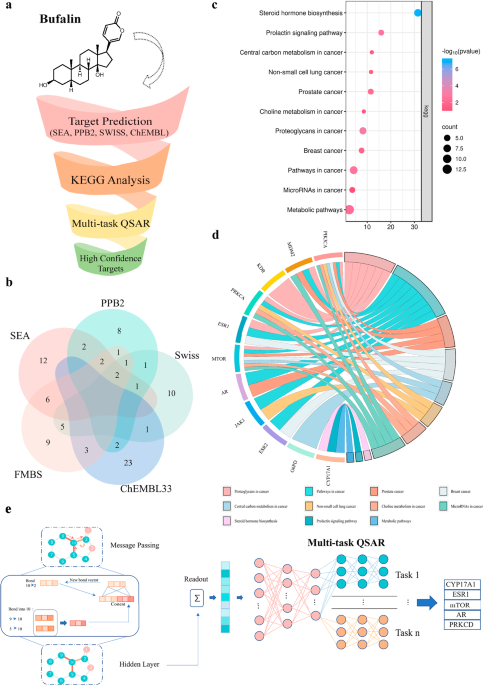
Target prediction of Bufalin based on an integrated multi-predictive strategy
To refine the target prediction range, we trained a deep learning model based on the Chemprop25 package using binding data from 11 targets sourced from ChEMBL and PubChem, with an average of 1187 compounds per target (Supplementary Table 2). The dataset was randomly split into an 80% training set and a 20% test set.
The model employs a Graph Neural Network (GNN) architecture specifically designed to learn molecular representations from graph-structured data. It consists of four primary components: (1) a shared local feature encoder that extracts atom and bond features across all tasks; (2) a directed message-passing process that propagates information along directed edges to generate atom embeddings; (3) an aggregation module that combines atom embeddings into a single molecular representation using sum or mean pooling; and (4) a feed-forward network (FFN) with task-specific multi-layer perceptron (MLP) layers to map molecular embeddings to target properties, enabling efficient multi-task predictions.
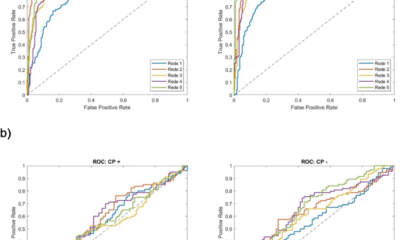
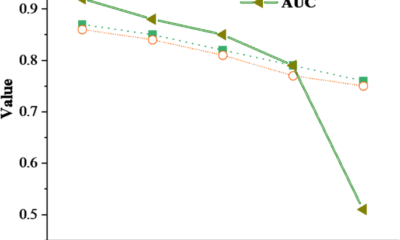
The model was trained with the Adam optimizer, incorporating learning rate scheduling, dropout regularization, and a model-ensembling strategy. Together with an advanced GNN architecture and a carefully curated dataset, this results in a robust and high-performing model, achieving a receiver operating characteristic area under the curve (ROC-AUC) of 0.94 on the test set (Supplementary Table 3). Finally, a default probability threshold of 0.8 was applied, ensuring that only targets meeting this performance criterion were selected.
Cell lines and culture
All cell lines were maintained at 37 °C in a humidified atmosphere of 5% CO2/95% air. MCF-7 cells were purchased from Cell Bank (Chinese Academy of Sciences, Beijing, China). The T47D and 293 T cell lines were purchased from Cell Bank (Chinese Academy of Sciences, Shanghai, China). The Tamoxifen-resistant cells LCC2 were derived from Wuhan University. MCF-7 and 293 T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Carlsbad, CA, USA) with 10% Fetal Bovine Serum and 1% penicillin streptomycin. T47D were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) with 10% Fetal Bovine Serum and 1% penicillin streptomycin. Tamoxifen-resistant LCC2 cells were cultured according to the literature52, briefly, LCC-2 cell lines were cultured in RPMI-1640 medium (10% Fetal Bovine Serum and 1% penicillin streptomycin). All cell lines were tested negative for mycoplasma contamination.
Reagents and Antibodies
Bufalin was purchased from APExBIO (USA). Fulvestrant, Estradiol (E2), and MG-132 were purchased from MedChemExpress (Monmouth Junction, NJ, USA). MLN4924 was purchased from TargetMol (Washington, USA). The recombinant Human CYP17A1 (cat. no. CSB-EP006392HU) and estrogen receptor (ESR1) (Cat. no. CSB-YP007830HU) proteins were purchased from CUSABIO. Recombinant human PKC delta protein (ab60844) was purchased from Abcam (Cambridge, UK). Antibodies targeting ERα (Cat. no. 8644, WB, 1:1000), LC3 (Cat. no. 12741, WB,1:1000), PARP (Cat. no. 9532, WB,1:1000), and Bcl-2 (Cat. no.15071, WB, 1:1000) were purchased from Cell Signaling Technology (Danvers, MA, USA). The ERα (cat. no. 84564-4-RR, IHC, 1:500, IF, 1:250), STUB1 (Cat. no. 68407-1-Ig, WB, 1:1000), and β-actin (Cat. no. 81115-1-RR, WB, 1:5000) antibodies were purchased from Proteintech (Chicago, IL, USA). The Flag (cat. no. M185, WB, 1:1000) and HA antibodies (cat. no. M180, WB, 1:1000) were purchased from MBL (Tokyo, Japan), while the antibody against ubiquitin (cat. no. sc-8017, WB, 1:200) was purchased from Santa Cruz Biotechnology. Anti-mouse and anti-rabbit secondary antibodies were purchased from Abiowell (Shanghai, China). Streptavidin FITC (Cat. no. 11-4317-87) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Magnetic streptavidin beads (cat. no. 22305-1) were purchased from Beaver (Suzhou, China). Lipofectamine 8000 was purchased from Beyotime Biotechnology (Shanghai, China). Lipofectamine™ RNAiMAX was purchased from Invitrogen. Protein A/G agarose beads (cat. no. 10121) were obtained from Santa Cruz Biotechnology, and the CCK-8 was purchased from Bimake (Shanghai, China). An enhanced chemiluminescence kit (cat. no. BL520A) was purchased from BioSharp (Shanghai, China).
Plasmid and siRNA transfection
Plasmids encoding wild-type and mutant ERα were obtained from Gene (Shanghai, China), while siRNAs targeting ESR1 and STUB1 were purchased from GenePharma (Suzhou, China). The target sequence of ESR1 siRNA was as follows: GCACCCUCUUGUAUUCCUATT (sense), UAGGAAUACAAGAGGGUGCTT (antisense). The target sequence of STUB1 siRNA was as follows: GCAGUCUGUGAAGGCGCACTT (sense), GUGCGCCUUCACAGACUGCTT (antisense). For siRNA transfection, the siRNA targeting ESR1 or STUB1 was incubated with Lipofectamine™ RNAiMAX in serum-free medium according to the manufacturer’s instructions. The plasmid was transfected using the Lipofectamine 8000 reagent in serum-free DMEM, according to the manufacturer’s instructions.
Western Blot
After treatment, the cells were washed twice with cold PBS and lysed on ice for 30 minutes in RIPA lysis (Abiowell) supplemented with a protease inhibitor cocktail (Biotool), followed by centrifugation at 12,000 g for 15 minutes at 4 °C. The protein concentration of the supernatant was determined using BCA. Proteins were resolved by SDS-PAGE and then transferred to PVDF membrane (Merck KGaA, Darmstadt, Germany). The PVDF membranes were blocked with 5% skim milk for 1 h at room temperature and then incubated with the respective antibodies at 4 °C for 14 h. After 14 h the PVDF membranes were washed thrice with PBST and incubated with a secondary antibody at room temperature for 1 h. The signals were detected by chemiluminescence assay using a ChemiDoc Touch (Bio-Rad).
Cell viability assays
Briefly, cells were seeded into 96-well plates at an appropriate density and allowed to adhere overnight. The following day, cells were treated with various concentrations of the indicated drug and incubated for the desired time period. Subsequently, 10 μL of CCK-8 solution was added to each well and the plates were incubated at 37 °C for 1-2 hours. After incubation, absorbance was measured at 450 nm using a microplate reader.
Colony forming assay
MCF-7, T47D, and LCC2 cells were seeded in 6-well plates, exposed to the indicated treatments, and cultured for approximately 15 days, and the medium was changed every 3 days. After treatment, the cells were fixed with 4% paraformaldehyde, stained with crystal violet for 24 h, washed with water, and colonies were counted.
5-Ethynyl-2’- deoxyuridine Assay (EdU)
After treatment with Bufalin, LCC2 cells were incubated with 5-ethynyl-2′-deoxyuridine (EdU; RiboBio, Guangzhou, China) for 2 hours at 37 °C, according to the manufacturer’s instructions. The cells were fixed with 4% paraformaldehyde at room temperature for 30 min, followed by treatment with 2 mg/mL glycine for 5 min. Next, the cells were permeabilized with 0.5% Triton X-100 for 10 min and stained with a 1× Apollo reaction cocktail for 30 min in the dark at room temperature. Finally, cell nuclei were counterstained with Hoechst 33342 for 30 min at room temperature. Images were captured using a fluorescence microscope.
Quantitative Real-time PCR
Total RNA was isolated from cells using the TRIzol reagent (CWBio, Taizhou, China), and reverse transcription was carried out using the PrimeScript RT reagent kit (TaKaRa, Japan) to generate complementary DNA (cDNA). Quantitative real-time PCR was conducted on a QuantStudio Real-Time PCR System (Life Technologies) using QuantStudio Design & Analysis Software v1.5.1. Gene expression levels were quantified using the standard 2−ΔΔCt method. The qPCR primer sets were: ESR1: GGGAAGTATGGCTATGGAATCTG (forward), TGGCTGGACACATATAGTCGTT (reverse); AGR2: AGAGCAGTTTGTCCTCCTCAA (forward), CAGGTTCGTAAGCATAGAGACG (reverse); CCND1: CAATGACCCCGCACGATTTC (forward), CATGGAGGGCGGATTGGAA (reverse); GREB1: TGGTCCGTAATGCACAAGGG (forward), CTGCGTTTAGTGAGGGGTGA (reverse); NRIP1: GGATCAGGTACTGCCGTTGAC (forward), CTGGACCATTACTTTGACAGGTG (reverse); PGR: TTATGGTGTCCTTACCTGTGGG (forward), GCGGATTTTATCAACGATGCAG (reverse); SIAH2: TCTTCGAGTGTCCGGTCTG (forward), CGGCATTGGTTACACACCAG (reverse), GAPDH: TGACATCAAGAAGGTGGTGAAGCAG (forward), GTGTCGCTGTTGAAGTCAGAGGAG (reverse).
Flow-cytometric analysis of apoptosis
After Bufalin treatment, the cells were collected and washed twice with cold PBS. Subsequently, 5 μL Annexin V-FITC and 5 μL propidium iodide (PI) staining buffer were added to the cells and incubated in the dark at room temperature. After 15 min, the stained cells were analyzed using FACS.
Immunofluorescence staining
MCF-7 cells treated with Biotin-Bufalin on glass coverslips were fixed in 4% paraformaldehyde for 30 min at room temperature and blocked with 5% bovine serum albumin (BSA) for 1 h. The fixed cells were then incubated with anti-ERα antibody and streptavidin FITC at 4 °C overnight, followed by Alexa Fluor 594 anti-rabbit IgG antibody. At the end of the incubation period, the cells’ nuclei were stained with DAPI, and the fluorescence signal was detected and captured using confocal microscopy.
Co-immunoprecipitation (Co-IP) assay
The 293 T cells were transiently transfected with Flag-ERα plasmid or HA-STUB1 and subjected to Bufalin treatment as indicated. After treatment, the cells were washed twice with PBS and lysed in mammalian protein extraction reagent buffer (cat. no. 78501; Thermo Scientific), supplemented with protease and phosphatase inhibitors for 30 min. Cell lysates were centrifuged, and supernatant was precleared with protein G agarose beads (Santa Cruz), then subjected to immunoprecipitation with indicated antibodies and protein A/G agarose beads at 4 °C overnight. The next day, the immunocomplexes were washed five times with PBS, and the binding proteins were eluted by 1 × SDS-PAGE loading buffer at 95 °C for 10 min. Bound proteins were identified using immunoblotting and western blot.
Biotin-pull down assay
After treatment, the cells were washed with PBS and lysed for 30 min in mammalian protein extraction (Thermo Scientific) with protease and phosphatase inhibitors. Following cell lysis, the supernatants were collected by centrifugation at 12,000 × g for 15 minutes at 4 °C. The streptavidin magnetic beads were pre-incubated with the cell lysate for 2 hours. Subsequently, 500 μg of clarified cell lysate was incubated with either D-Biotin or Biotin-Bufalin overnight at 4 °C for target protein capture. The following day, the mixture containing biotin and cell lysate was coupled with streptavidin magnetic beads (Beaver) for 2 hours at room temperature. The magnetic beads were then collected using a magnetic rack and washed six times to remove non-specifically bound proteins. Bound proteins were eluted by boiling in 1× SDS-PAGE loading buffer at 95 °C for 10 minutes, followed by detection via western blot analysis.
Animal studies
Briefly, the Tamoxifen-resistant cells LCC2 (2 × 106 cells) were subcutaneously injected into 4-week-old female nude mice (Hunan Slack Jingda Laboratory Animal Co., Ltd.). Tumor sizes were measured on different days after inoculation and calculated using the formula V = lw2π/6, where l is the length and w is the width. When the tumors were palpable, the mice were randomly divided into designated groups and received the indicated treatments. The tumor volume was measured using a Vernier caliper every two days.
Immunohistochemistry (IHC)
The human cancer tissue specimens from patients with Tamoxifen treatment and recurrence, and animal tissue were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. After deparaffinization, antigen retrieval was performed using a citric acid buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and following blocked with the application of normal serum. Sections were then incubated overnight at 4 °C with the antibodies against ERα and Ki67, followed by HRP-conjugated secondary antibodies. The signal was developed using DAB, and the nuclei were counterstained with hematoxylin. Immunohistochemical staining was performed according to the manufacturer’s protocol.
Protein-ligand binding conformation modeling
The conformation of the protein-ligand complex was modeled using Schrödinger software (version 2022.1). The structure of Bufalin was obtained from PubChem, and ligand conformations were generated using LigPrep. The crystal structure of the ESR1 protein was obtained from the Protein Data Bank (PDB ID: 3ERT)63. The Glide SP protocol was employed to generate the optimal conformation of the protein-ligand complex.
Protein-protein docking conformation modeling
The structures of STUB1, TRIM56, RNF181, RNF2, and ESR1 proteins were obtained from the Protein Data Bank and Alphafold 3, and subjected to 15 repeats of optimization using the relax module of Rosetta (version 3.5.1)64. Ten conformations were generated from each optimization, and the top-scoring conformation from Rosetta was used for subsequent protein-protein docking. Initial protein-protein docking was performed using the ClusPro server65. Complex conformations with incorrect STUB1, TRIM56, RNF181, and RNF2 binding modes were excluded from the ClusPro docking results. These conformations were subjected to structural refinement using the docking protocol of Rosetta. All generated conformations were ranked according to their Rosetta scores.
Molecular dynamic simulations
Molecular simulations of ESR1-Bufalin, ESR1-STUB1, and ESR1-Bufalin-STUB1 complexes were conducted using Amber22 software. Proteins and ligands were parameterized using Amber ff19SB66 and GAFF267 force fields, respectively. The complex systems were solvated in a TIP3P water box extending 10 Å from the protein. Chloride and sodium ions were then added to neutralize the system. Subsequently, energy minimization was performed for up to 20,000 steps. The systems were heated from 0 K to 298.15 K over 100 ps and the pressure was increased to atmospheric pressure over another 100 ps, with a time step of 1 fs during the heating and pressurization processes. Finally, a 200 ns production classical MD simulation was conducted with a time step of 2 fs. Trajectory analysis was performed using CPPTRAJ.
MM-PBSA and alanine scanning mutations analysis
MM-PBSA binding free energy calculations were performed using MMPBSA.py from AmberTools202368, using only the last 100 ns of the trajectory. We extracted 100 frames from the trajectory for subsequent energy calculations. In addition, residual energy decomposition was performed for the Bufalin-ESR1 complex. Alanine scanning mutations69 were conducted on the top ten residues from the energy decomposition analysis.
Surface Plasmon Resonance (SPR) assay
After storage at -80 °C, Bufalin and the recombinant proteins CYP17A1, ESR1, and PKC delta were allowed to equilibrate to room temperature. Bufalin was diluted with DMSO to the appropriate concentration for spotting and used as the immobilized phase. The working solution was printed onto a 3D photocrosslinkable sensor chip using the BioDot™ AD1520 microarray printer, with four nonadjacent replicate spots per compound. The printed chips were vacuum-dried and then subjected to UV-induced photocrosslinking using a crosslinking instrument. Following crosslinking, the chips were sequentially washed on a shaker with DMF, ethanol (EtOH), and deionized water for 15 minutes each. The recombinant protein samples were diluted to generate five concentration gradients: 200 nM, 400 nM, 800 nM, 1600 nM, and 3200 nM, and were injected over the chip surface. During interaction analysis, the protein analytes were passed over the chip surface at a flow rate of 0.5 μL/s. Each association phase lasted 600 seconds, followed by a 360-second dissociation phase. After each binding cycle, the chip surface was regenerated with 10 mM glycine-HCl (pH 2.0) at a flow rate of 2 μL/s to remove the bound analytes.
Patient-derived organoid
Tissue specimens from patients who relapsed after Tamoxifen therapy were processed for organoid culture according to previous protocols70,71. Samples were obtained from the Xiangya Hospital of Central South University (Changsha, China), and written informed consent was obtained from all participants prior to collection. Tissue samples were enzymatically digested into single-cell suspensions using collagenase (Sigma) and subsequently cultured in a 3D environment composed of 50% chilled Matrigel (Corning). After 5 days of culture, the resulting organoids were dissociated into single-cell suspensions, seeded into 384-well plates, and treated with Bufalin. After 4 days of treatment, cell viability was assessed using CellTiter-Glo 3D Reagent (Promega) according to the manufacturer’s instructions.
Organoid live/dead cell staining
Live/dead organoid cell staining was performed as previously described72. Following treatment, the samples were washed twice with cold PBS and subsequently stained with Calcein-AM and propidium iodide (PI) at 37 °C for 20 minutes in the dark. Finally, the images were captured using a fluorescence microscope.
Statistics and reproducibility
We analyzed the data using GraphPad Prism 9.0 software. All samples represent biological replicates, and the statistical measurements are presented as mean ± SD as specified in each figure. No statistical method was used to predetermine sample size. No data were excluded from the analyses. The experiments were randomized. The Investigators were blinded to allocation during experiments and outcome assessment. Statistical analyses were performed using one-way or two-way ANOVA, depending on the experimental design. A P-value < 0.05 was considered statistically significant. The P values are given in the figures. All representative experiments are repeated at least three times independently with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
AI Research
Attorneys general warn OpenAI ‘harm to children will not be tolerated’

California Attorney General Rob Bonta and Delaware Attorney General Kathy Jennings met with and sent an open letter to OpenAI to express their concerns over the safety of ChatGPT, particularly for children and teens.
The warning comes a week after Bonta and 44 other attorneys general sent a letter to 12 of the top AI companies, following reports of sexually inappropriate interactions between AI chatbots and children.
“Since the issuance of that letter, we learned of the heartbreaking death by suicide of one young Californian after he had prolonged interactions with an OpenAI chatbot, as well as a similarly disturbing murder-suicide in Connecticut,” Bonta and Jennings write. “Whatever safeguards were in place did not work.”
The two state officials are currently investigating OpenAI’s proposed restructuring into a for-profit entity to ensure that the mission of the nonprofit remains intact. That mission “includes ensuring that artificial intelligence is deployed safely” and building artificial general intelligence (AGI) to benefit all humanity, “including children,” per the letter.
“Before we get to benefiting, we need to ensure that adequate safety measures are in place to not harm,” the letter continues. “It is our shared view that OpenAI and the industry at large are not where they need to be in ensuring safety in AI products’ development and deployment. As Attorneys General, public safety is one of our core missions. As we continue our dialogue related to OpenAI’s recapitalization plan, we must work to accelerate and amplify safety as a governing force in the future of this powerful technology.”
Bonta and Jennings have asked for more information about OpenAI’s current safety precautions and governance, and said they expect the company to take immediate remedial measures where appropriate.
TechCrunch has reached out to OpenAI for comment.
Techcrunch event
San Francisco
|
October 27-29, 2025
AI Research
Ketryx Closes $39M Series B Round to Power the Future of Regulated Artificial Intelligence for Life Sciences

Insider Brief
- Ketryx raised $39M Series B led by Transformation Capital, with participation from Lightspeed, MIT’s E14 Fund, Ubiquity Ventures, and 53 Stations, bringing total funding to over $55M; Vinay Shah of Transformation Capital joins the board.
- Its AI-native compliance platform automates validation, traceability, and regulatory workflows (FDA/EU MDR-ready), enabling life sciences teams to achieve up to 90% faster documentation and 10x quicker release cycles without sacrificing safety.
- Already used by three of the top five global medtech companies and innovators like DeepHealth and Heartflow, Ketryx is positioning itself as the key AI infrastructure layer for regulated product development in healthcare and beyond.
Ketryx, the AI-powered compliance platform helping life sciences companies ship safer products faster, has announced a $39 million Series B led by Transformation Capital, with participation from existing investors including Lightspeed Venture Partners, MIT’s E14 Fund, Ubiquity Ventures, and 53 Stations. This latest round brings the company’s total funding to over $55 million, and Vinay Shah, Partner and Founding Team Member at Transformation Capital, will join Ketryx’s board.
Ketryx is solving one of the most difficult challenges in the life sciences: the need to accelerate product innovation without compromising safety or compliance. This challenge is more urgent than ever with teams racing to incorporate AI into regulated workflows and products.
“I’ve spent the last decade at the intersection of AI and life sciences, watching it evolve from an emerging tool to a critical application for patients,” said Erez Kaminski, CEO and founder of Ketryx. “It’s now time to accelerate adoption and ensure AI is safe, reliable, and ready for regulated environments.”
Life sciences teams are struggling to balance rigorous compliance requirements amid the rapidly accelerating pace of innovation. While cloud-based tools and rapidly evolving LLMs are transforming what’s possible, these regulated teams are still operating on infrastructure not designed for this velocity of change.
Ketryx is an AI-native compliance platform built to meet this challenge. It automates validation, traceability, and regulatory workflows — including FDA/EU MDR-ready documentation — across the product development lifecycle to help teams release safer products faster. Customers report up to a 90% reduction in documentation time and over 10x faster release cycles.
“In Medtech, long-term success depends on balancing innovation with the uncompromising demands of safety and compliance,” said Bill Hawkins, former CEO of Medtronic and new Ketryx investor. “This balance has historically been hard to achieve. Ketryx has built the infrastructure that allows both to advance together. Their ability to deliver this level of rigor at true enterprise scale is why I’m proud to support them as they shape the future of regulated software.”
The company’s platform is built for the enterprise and already used by three of the top five global medtech companies, several Fortune 500 organizations, and AI-powered companies such as DeepHealth, Heartflow, and Aignostics. With adoption accelerating, Ketryx is emerging as the key AI infrastructure layer for product development in regulated industries.
“Medtech teams are leading the way in applying artificial intelligence to improve patient outcomes, creating products that meet the highest safety and regulatory standards,” said Vinay Shah, Partner and Founding Team Member at Transformation Capital. “In our diligence, Fortune 500 giants and fast-growing innovators consistently praised Ketryx for proving that compliance can accelerate, rather than slow, technological progress. We believe Ketryx is defining the future of regulated infrastructure across industries and are proud to back them in their next stage of growth.”
Kaminski continued, “Having Transformation Capital, the pre-eminent digital health VC & growth equity firm, as our lead partner, gives us more than just capital. They understand exactly what it takes to build and scale healthcare technology companies. With their backing and industry connections, we’re continuing our global expansion, accelerating our product roadmap, and hiring rapidly in both Boston and Austria.”
With real-time traceability and documentation, Ketryx brings zero-lag compliance to the heart of product development, empowering teams to release more products, more safely, and faster than ever before.
About Ketryx
Ketryx transforms the product lifecycle of life science teams to deliver safer products, faster. Trusted by three of the world’s top five medical device manufacturers, its AI-powered compliance platform overlays existing tools to automate documentation, create traceability, and accelerate release cycles — without disrupting existing workflows. Ketryx AI Agents cut manual work by 90 percent and close compliance gaps, elevating speed and quality across the entire product lifecycle. For more information, visit www.ketryx.com.
AI Research
How could an OpenAI partnership with Broadcom shake up Silicon Valley’s chip hierarchy?

Broadcom Inc. is helping OpenAI design and produce an artificial intelligence accelerator from 2026, getting into a lucrative sphere dominated by Nvidia Corp. Its shares jumped by the most since April.
The two firms plan to ship the first chips in that lineup starting next year, a person familiar with the matter said, asking to remain anonymous discussing a private deal. OpenAI will initially use the chip for its own internal purposes, the Financial Times reported earlier.
Broadcom’s shares surged as much as 16% in New York trading on Friday, adding more than $200 billion to the company’s market value. Nvidia’s stock was down as much as 4.3% at $164.22, its biggest intraday decline since May.
Chief Executive Officer Hock Tan made veiled references to that partnership on Thursday when he said Broadcom had secured a new client for its custom accelerator business. Tan said the company has secured more than $10 billion in orders from the new customer, which the person identified as OpenAI.
Accelerators are essential to the development of AI at big tech firms from Meta Platforms Inc. to Microsoft Corp. Bloomberg News has previously reported that OpenAI and Broadcom were working on an inference chip design, intended to run or operate artificial intelligence services after they had been trained.
“Last quarter, one of these prospects released production orders to Broadcom,” Tan said, without naming the customer.
Broadcom is among the chip designers benefiting from a post-ChatGPT boom in AI development, in which companies and startups from the US to China are spending billions to build data centers, train new models and research breakthroughs in a pivotal new technology. On Thursday, Tan told investors the chipmaker’s outlook will improve “significantly” in fiscal 2026, helping allay concerns about slowing growth.
Tan had previously said that AI revenue for 2026 would show growth similar to the current year — a rate of 50% to 60%. Now, with a new customer that he said has “immediate and pretty substantial demand,” the rate will accelerate in a way that will be “fairly material,” Tan said.
“We now expect the outlook for fiscal 2026 AI revenue to improve significantly from what we had indicated last quarter,” he said.
Broadcom’s quarterly results initially drew a tepid reaction from investors, a sign they were anticipating a bigger payoff from the AI boom. After fluctuating in the wake of the report, the stock gained more than 3% during the conference call.
Sales will be about $17.4 billion in the fiscal fourth quarter, which runs through October, the company said in an earlier statement. Analysts had projected $17.05 billion on average, though some estimates topped $18 billion, according to data compiled by Bloomberg.
Expectations were high heading into the earnings report. Broadcom shares more than doubled since hitting a low in April, adding about $730 billion to the company’s market value and making them the third-best performer in the Nasdaq 100 Index.
Investors have been looking for signs that tech spending remains strong. Last week, Nvidia gave an underwhelming revenue forecast, sparking fears of a bubble in the artificial intelligence industry.
Though Broadcom hasn’t experienced Nvidia’s runaway sales growth, it is seen as a key AI beneficiary. Customers developing and running artificial intelligence models rely on its custom-designed chips and networking equipment to handle the load. The shares had been up 32% for the year.
During the call, Tan said he and the board have agreed that he will stay as Broadcom CEO until 2030 “at least.”
In the third quarter ended Aug. 3, sales rose 22% to almost $16 billion. Profit, excluding some items, was $1.69 a share. Analysts had estimated revenue of about $15.8 billion and earnings of $1.67 a share.
Sales of AI semiconductors were $5.2 billion, compared with an estimate of $5.11 billion. The company expects revenue from that category to reach $6.2 billion in the fourth quarter. Analysts projected $5.82 billion.
Other AI-focused chipmakers have stumbled in recent days. Shares of Marvell Technology Inc., a close Broadcom competitor in the market for custom semiconductors, plunged 19% on Friday after the company’s data center revenue missed estimates.
Broadcom’s Tan has been upgrading the company’s networking equipment to better transfer information between the pricey graphics chips at the heart of AI data centers. As his latest comments suggest, Broadcom is also making progress finding customers who want custom-designed chips for AI tasks.
Tan has used years of acquisitions to turn Broadcom into a sprawling software and hardware giant. In addition to the AI work, the Palo Alto, California-based company makes connectivity components for Apple Inc.’s iPhone and sells virtualization software for running networks.
Bass writes for Bloomberg.
-

 Business1 week ago
Business1 week agoThe Guardian view on Trump and the Fed: independence is no substitute for accountability | Editorial
-
Tools & Platforms4 weeks ago
Building Trust in Military AI Starts with Opening the Black Box – War on the Rocks
-

 Ethics & Policy1 month ago
Ethics & Policy1 month agoSDAIA Supports Saudi Arabia’s Leadership in Shaping Global AI Ethics, Policy, and Research – وكالة الأنباء السعودية
-

 Events & Conferences4 months ago
Events & Conferences4 months agoJourney to 1000 models: Scaling Instagram’s recommendation system
-

 Jobs & Careers2 months ago
Jobs & Careers2 months agoMumbai-based Perplexity Alternative Has 60k+ Users Without Funding
-

 Education2 months ago
Education2 months agoVEX Robotics launches AI-powered classroom robotics system
-

 Funding & Business2 months ago
Funding & Business2 months agoKayak and Expedia race to build AI travel agents that turn social posts into itineraries
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoHappy 4th of July! 🎆 Made with Veo 3 in Gemini
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoOpenAI 🤝 @teamganassi
-

 Education2 months ago
Education2 months agoMacron says UK and France have duty to tackle illegal migration ‘with humanity, solidarity and firmness’ – UK politics live | Politics