AI Research
Donald Trump is Helping China in the AI Race. Why?

Nvidia has ordered 300,000 new top-line computer chips from the Taiwan Semiconductor Manufacturing Company (TSMC) following a huge boost in demand from China.
The agreement over the H20 chipsets, which are some of the most advanced technology being produced in Taiwan’s industry-leading factories, comes in the same month that Trump reversed a ban that stopped Nvidia from selling to China over security concerns.
Newsweek contacted the Chinese Embassy and the White House for comment on this story via email.
Why It Matters
The January launch of DeepSeek, a Chinese-made AI model, , sparked what may have called a “cold war” over artificial intelligence development. Researchers developed DeepSeek with a fraction of the resources but managed to produce an AI capable of rivaling ChatGPT.
In April, the Trump administration barred top U.S. suppliers, like Nvidia, from selling topline silicon to China over “national security concerns.” The ban mirrored the 2022 CHIPS Act passed by former President Joe Biden, which increased semiconductor manufacturing in the U.S. while also clamping down on chip companies investing in China and Russia, the biggest competitors in the industry.
What To Know
The White House reversed its ban after barely three months, giving Nvidia the all-clear in July to resume sales with China. Nvidia founder and CEO Jensen Huang on July 14 said the U.S. government had assured him it would restore the licenses to sell H20s in China.
“General-purpose, open-source research and foundation models are the backbone of AI innovation,” Huang said. “We believe that every civil model should run best on the U.S. technology stack, encouraging nations worldwide to choose America.”
Half a month later, and Chinese demand for Nvidia chips has surged, with the U.S. company looking to replenish its stock with an order of 300,000 H20 chipsets from TSMC, one of the largest manufacturers in the world.
Getty Images
The return of Nvidia chips to the Chinese market will be a boon to the country’s rapidly expanding AI industry, but there’s concern that the U.S. could fall behind in an AI “race”, as OpenAI CEO Sam Altman describes it, using U.S. chips to do so.
In May, a report from the Georgetown University’s Center for Security and Emerging Technology shared with Newsweek found that two of China’s leading AI institutes, headquartered in Beijing, have established branches in Wuhan to cooperate on sophisticated alternatives to the large generative AI models.
The report described the new labs’ aim to “springboard to artificial general intelligence”, overtaking the U.S. by focusing on other forms of AI as opposed to the western focus on large statistical models.
What People Are Saying
Alexandra Mousavizadeh, CEO of Evident and creator of the Global AI Index, told Newsweek that there were two different approaches to China’s AI development: “You can continue to try and contain access to chips and close the walls off. While you’re doing that, you’re doubling down on investment into data infrastructure, supporting the development of AI in the U.S. and being first in that race,
“Or you open up completely and you say, ‘Look, it’s to the benefit of all that everyone has access to everything, because the collaboration between Europe, the U.S. and China in the past has been what has led to the ability to get to where we are today.’
A spokesperson for the Chinese Embassy told Newsweek: “China has consistently advocated that the development of artificial intelligence should adhere to principles that are human-centered and promote benevolence.
“China believes that AI development should be fair and inclusive, ensuring that all countries equally enjoy the benefits brought by AI, and has no intention of seeking dominance in this field.”
What Happens Next
China will continue to be able to purchase from Nvidia and other U.S. chip manufacturers unless the White House alters its policy.
AI Research
Indonesia unveils national AI roadmap
Artificial Intelligence (AI) could help Indonesia achieve its vision of Golden Indonesia 2045 with the right strategy and governance, according to Minister of Communication and Digital Affairs, Meutya Hafid.
Stating this in her forward to Indonesia’s National AI Roadmap White Paper, she said the AI roadmap would provide policy direction to accelerate AI ecosystem development to ensure the country was not to be left behind in a field increasingly dominated by advanced countries and global tech giants.
The White Paper, drafted by the AI Roadmap Task Force, a 443-member body representing government, academia, industry, civil society, and the media, was launched by the Ministry of Communication and Digital in early August.
It has been envisaged as a strategic document that would serve as the country’s reference for adopting and developing AI technology in a more focused, inclusive, and ethical manner. The document has been circulated for public consultation to gather wider input from stakeholders.
This initiative builds on the National AI Strategy 2020-2045, which was an initial framework developed by the Collaborative Research and Industrial Innovation in AI (KORIKA), an organisation formed by scientists, technocrats and industry leaders to accelerate the AI ecosystem in Indonesia.
However, that strategy has struggled to keep up with the rapid breakthroughs in generative AI (GenAI) since late 2022.
Three major action plans
The national AI roadmap outlines three main action plans: AI ecosystems, AI development priorities, and AI financing – all anchored in ethical guidance and regulation.
This roadmap also breaks down the action plan into three-time horizons: short term (2025-2027), medium term (2028-2035) and long term (2035-2045).
To subscribe to the GovInsider bulletin, click here.
-1756728192656.jpg)
Indonesia’s AI ecosystem development would focus on three main pillars.
The first pillar was talent development.
Indonesia aimed to nurture a large pool of skilled professionals who could both use and create AI innovation.
The roadmap sets an ambitious target of producing 100,000 AI talents annually. Around 30 per cent would be developers, divided further into AI specialists (30 per cent) and practitioners (70 per cent), and the remaining 70 per cent would be AI end-users.
The government also aimed to ensure 20 million citizens are AI-literate by 2029.
The next pillar was research and industrial innovation.
The roadmap emphasised advanced, relevant, and sustainable AI research that delivered real benefits to society.
To achieve this, the government would encourage agencies, universities, and industries to strengthen AI programmes in priority sectors.
A cross-sectoral open sandbox platform would also be developed to support experimentation and collaboration.
The last pillar in Indonesia’s AI ecosystem was infrastructure and data.
To foster domestic AI innovation, the government planned to expand digital infrastructure, including high-performance computing, GPUs/TPUs, and a national cloud hosted in sovereign data centres to ensure secure and regulated data management.
The white paper also outlined plans to promote the development of green data centres through public–private partnerships.
Strategic priorities in AI development
The roadmap focuses on developing AI for strategic use cases, ensuring that AI adoption delivers meaningful and sustainable impact.
These priorities closely align with the country’s national development agenda and President Prabowo’s Asta Cita vision.
The priority sectors for AI include food security, healthcare, education, economy and finance, bureaucratic reform, politics and security, energy, environment, housing, transport and logistics, as well as arts, culture, and the creative economy.
Public services were also identified as an immediate priority for the 2025–2027 term. In healthcare, AI would be applied for early disease detection, remote patient monitoring, and optimising the distribution of medicines and vaccines.
In education, the focus would be on adaptive learning and digital platforms for personalised teaching materials. The government also plans to develop automated evaluation systems to ease assessment processes in schools.
In governance, AI applications would centre on intelligent chatbots for public services and data-driven policy analytics.
For transport and mobility, development would be directed towards smart traffic systems, public transport management, and the optimisation of national logistics.
Financing the national AI agenda
The roadmap outlined a phased financing strategy, combining state budget allocations, private sector contributions, and external partnerships through bilateral and multilateral collaborations.
Over the next two decades, the government aimed to establish a sustainable financing ecosystem driven by industry participation and international investment. To achieve this, Indonesia will expand fiscal incentives to encourage AI-related investments.
A notable feature of the roadmap was the role of Danantara, Indonesia’s newly established sovereign wealth fund, which has been tasked with spearheading AI financing.
Danantara would design innovative financial instruments, establish a Sovereign AI Fund, and develop blended financing models for the country’s strategic AI projects.
In the initial phase, financing would target fundamental research, pilot projects in the public sector, and the development of data and computing infrastructure.
Subsequent stages would extend funding to industries, research institutions, universities, and domestic AI start-ups, with the goal of strengthening Indonesia’s AI ecosystem and boosting its global competitiveness.
AI Research
MAIA platform for routine clinical testing: an artificial intelligence embryo selection tool developed to assist embryologists
Graham, M. E. et al. Assisted reproductive technology: Short- and long-term outcomes. Dev. Med. Child. Neurol. 65, 38–49 (2023).
Jiang, V. S. & Bormann, C. L. Artificial intelligence in the in vitro fertilization laboratory: a review of advancements over the last decade. Fertil. Steril. 120, 17–23 (2023).
Devine, K. et al. Single vitrified blastocyst transfer maximizes liveborn children per embryo while minimizing preterm birth. Fertil. Steril. 103, 1454–1460 (2015).
Tiitinen, A. Single embryo transfer: why and how to identify the embryo with the best developmental potential. Best Pract. Res. Clin. Endocrinol. Metab. 33, 77–88 (2019).
Glatstein, I., Chavez-Badiola, A. & Curchoe, C. L. New frontiers in embryo selection. J. Assist. Reprod. Genet. 40, 223–234 (2023).
Gardner, D. K. & Schoolcraft, W. B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynaecol. 11, 307–311 (1999).
Sciorio, R. & Meseguer, M. Focus on time-lapse analysis: blastocyst collapse and morphometric assessment as new features of embryo viability. Reprod. BioMed. Online. 43, 821–832 (2021).
Sundvall, L., Ingerslev, H. J., Knudsen, U. B. & Kirkegaard, K. Inter- and intra-observer variability of time-lapse annotations. Hum. Reprod. 28, 3215–3221 (2013).
Gallego, R. D., Remohí, J. & Meseguer, M. Time-lapse imaging: the state of the Art. Biol. Reprod. 101, 1146–1154 (2019).
VerMilyea, M. D. et al. Computer-automated time-lapse analysis results correlate with embryo implantation and clinical pregnancy: a blinded, multi-centre study. Reprod. Biomed. Online. 29, 729–736 (2014).
Chéles, D. S., Molin, E. A. D., Rocha, J. C. & Nogueira, M. F. G. Mining of variables from embryo morphokinetics, blastocyst’s morphology and patient parameters: an approach to predict the live birth in the assisted reproduction service. JBRA Assist. Reprod. 24, 470–479 (2020).
Rocha, C., Nogueira, M. G., Zaninovic, N. & Hickman, C. Is AI assessment of morphokinetic data and digital image analysis from time-lapse culture predictive of implantation potential of human embryos? Fertil. Steril. 110, e373 (2018).
Zaninovic, N. et al. Application of artificial intelligence technology to increase the efficacy of embryo selection and prediction of live birth using human blastocysts cultured in a time-lapse incubator. Fertil. Steril. 110, e372–e373 (2018).
Alegre, L. et al. First application of artificial neuronal networks for human live birth prediction on Geri time-lapse monitoring system blastocyst images. Fertil. Steril. 114, e140 (2020).
Bori, L. et al. An artificial intelligence model based on the proteomic profile of euploid embryos and blastocyst morphology: a preliminary study. Reprod. BioMed. Online. 42, 340–350 (2021).
Chéles, D. S. et al. An image processing protocol to extract variables predictive of human embryo fitness for assisted reproduction. Appl. Sci. 12, 3531 (2022).
Jacobs, C. K. et al. Embryologists versus artificial intelligence: predicting clinical pregnancy out of a transferred embryo who performs it better? Fertil. Steril. 118, e81–e82 (2022).
Lorenzon, A. et al. P-211 development of an artificial intelligence software with consistent laboratory data from a single IVF center: performance of a new interface to predict clinical pregnancy. Hum. Reprod. 39, deae108.581 (2024).
Fernandez, E. I. et al. Artificial intelligence in the IVF laboratory: overview through the application of different types of algorithms for the classification of reproductive data. J. Assist. Reprod. Genet. 37, 2359–2376 (2020).
Mendizabal-Ruiz, G. et al. Computer software (SiD) assisted real-time single sperm selection associated with fertilization and blastocyst formation. Reprod. BioMed. Online. 45, 703–711 (2022).
Fjeldstad, J. et al. Segmentation of mature human oocytes provides interpretable and improved blastocyst outcome predictions by a machine learning model. Sci. Rep. 14, 10569 (2024).
Khosravi, P. et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit. Med. 2, 21 (2019).
Hickman, C. et al. Inner cell mass surface area automatically detected using Chloe eq™(fairtility), an ai-based embryology support tool, is associated with embryo grading, embryo ranking, ploidy and live birth outcome. Fertil. Steril. 118, e79 (2022).
Tran, D., Cooke, S., Illingworth, P. J. & Gardner, D. K. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum. Reprod. 34, 1011–1018 (2019).
Rajendran, S. et al. Automatic ploidy prediction and quality assessment of human blastocysts using time-lapse imaging. Nat. Commun. 15, 7756 (2024).
Bormann, C. L. et al. Consistency and objectivity of automated embryo assessments using deep neural networks. Fertil. Steril. 113, 781–787e1 (2020).
Kragh, M. F. & Karstoft, H. Embryo selection with artificial intelligence: how to evaluate and compare methods? J. Assist. Reprod. Genet. 38, 1675–1689 (2021).
Cromack, S. C., Lew, A. M., Bazzetta, S. E., Xu, S. & Walter, J. R. The perception of artificial intelligence and infertility care among patients undergoing fertility treatment. J. Assist. Reprod. Genet. https://doi.org/10.1007/s10815-024-03382-5 (2025).
Fröhlich, H. et al. From hype to reality: data science enabling personalized medicine. BMC Med. 16, 150 (2018).
Zhu, J. et al. External validation of a model for selecting day 3 embryos for transfer based upon deep learning and time-lapse imaging. Reprod. BioMed. Online. 47, 103242 (2023).
Yelke, H. K. et al. O-007 Simplifying the complexity of time-lapse decisions with AI: CHLOE (Fairtility) can automatically annotate morphokinetics and predict blastulation (at 30hpi), pregnancy and ongoing clinical pregnancy. Hum. Reprod. 37, deac104.007 (2022).
Papatheodorou, A. et al. Clinical and practical validation of an end-to-end artificial intelligence (AI)-driven fertility management platform in a real-world clinical setting. Reprod. BioMed. Online. 45, e44–e45 (2022).
Salih, M. et al. Embryo selection through artificial intelligence versus embryologists: a systematic review. Hum. Reprod. Open hoad031 (2023).
Nunes, K. et al. Admixture’s impact on Brazilian population evolution and health. Science. 388(6748), eadl3564 (2025).
Jackson-Bey, T. et al. Systematic review of Racial and ethnic disparities in reproductive endocrinology and infertility: where do we stand today? F&S Reviews. 2, 169–188 (2021).
Kassi, L. A. et al. Body mass index, not race, May be associated with an alteration in early embryo morphokinetics during in vitro fertilization. J. Assist. Reprod. Genet. 38, 3091–3098 (2021).
Pena, S. D. J., Bastos-Rodrigues, L., Pimenta, J. R. & Bydlowski, S. P. DNA tests probe the genomic ancestry of Brazilians. Braz J. Med. Biol. Res. 42, 870–876 (2009).
Fraga, A. M. et al. Establishment of a Brazilian line of human embryonic stem cells in defined medium: implications for cell therapy in an ethnically diverse population. Cell. Transpl. 20, 431–440 (2011).
Amin, F. & Mahmoud, M. Confusion matrix in binary classification problems: a step-by-step tutorial. J. Eng. Res. 6, 0–0 (2022).
Magdi, Y. et al. Effect of embryo selection based morphokinetics on IVF/ICSI outcomes: evidence from a systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 300, 1479–1490 (2019).
Guo, Y. H., Liu, Y., Qi, L., Song, W. Y. & Jin, H. X. Can time-lapse incubation and monitoring be beneficial to assisted reproduction technology outcomes? A randomized controlled trial using day 3 double embryo transfer. Front. Physiol. 12, 794601 (2022).
Giménez, C., Conversa, L., Murria, L. & Meseguer, M. Time-lapse imaging: morphokinetic analysis of in vitro fertilization outcomes. Fertil. Steril. 120, 228–227 (2023).
Vitrolife EmbryoScope + time-lapse system. (2023). https://www.vitrolife.com/products/time-lapse-systems/embryoscopeplus-time-lapse-system/.
Lagalla, C. et al. A quantitative approach to blastocyst quality evaluation: morphometric analysis and related IVF outcomes. J. Assist. Reprod. Genet. 32, 705–712 (2015).
Rocha, J. C. et al. A method based on artificial intelligence to fully automatize the evaluation of bovine blastocyst images. Sci. Rep. 7, 7659 (2017).
Chavez-Badiola, A. et al. Predicting pregnancy test results after embryo transfer by image feature extraction and analysis using machine learning. Sci. Rep. 10, 4394 (2020).
Matos, F. D., Rocha, J. C. & Nogueira, M. F. G. A method using artificial neural networks to morphologically assess mouse blastocyst quality. J. Anim. Sci. Technol. 56, 15 (2014).
Wang, S., Zhou, C., Zhang, D., Chen, L. & Sun, H. A deep learning framework design for automatic blastocyst evaluation with multifocal images. IEEE Access. 9, 18927–18934 (2021).
Berntsen, J., Rimestad, J., Lassen, J. T., Tran, D. & Kragh, M. F. Robust and generalizable embryo selection based on artificial intelligence and time-lapse image sequences. PLoS One. 17, e0262661 (2022).
Fruchter-Goldmeier, Y. et al. An artificial intelligence algorithm for automated blastocyst morphometric parameters demonstrates a positive association with implantation potential. Sci. Rep. 13, 14617 (2023).
Illingworth, P. J. et al. Deep learning versus manual morphology-based embryo selection in IVF: a randomized, double-blind noninferiority trial. Nat. Med. 30, 3114–3120 (2024).
Kanakasabapathy, M. K. et al. Development and evaluation of inexpensive automated deep learning-based imaging systems for embryology. Lab. Chip. 19, 4139–4145 (2019).
Loewke, K. et al. Characterization of an artificial intelligence model for ranking static images of blastocyst stage embryos. Fertil. Steril. 117, 528–535 (2022).
Hengstschläger, M. Artificial intelligence as a door opener for a new era of human reproduction. Hum. Reprod. Open hoad043 (2023).
Lassen Theilgaard, J., Fly Kragh, M., Rimestad, J., Nygård Johansen, M. & Berntsen, J. Development and validation of deep learning based embryo selection across multiple days of transfer. Sci. Rep. 13 (1), 4235 (2023).
Lozano, M. et al. P-301 Assessment of ongoing clinical outcomes prediction of an AI system on retrospective SET data, Human Reprod. 38(Issue Supplement_1), dead093.659. (2023).
Collins, G. S. et al. TRIPOD + AI statement: updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 385, e078378 (2024).
Abdolrasol, M. G. M. et al. Artificial neural networks based optimization techniques: a review. Electronics 10, 2689 (2021).
Yuzer, E. O. & Bozkurt, A. Instant solar irradiation forecasting for solar power plants using different ANN algorithms and network models. Electr. Eng. 106, 3671–3689 (2024).
Guariso, G. & Sangiorgio, M. Improving the performance of multiobjective genetic algorithms: an elitism-based approach. Information 11, 587 (2020).
García-Pascual, C. M. et al. Optimized NGS approach for detection of aneuploidies and mosaicism in PGT-A and imbalances in PGT-SR. Genes 11, 724 (2020).
AI Research
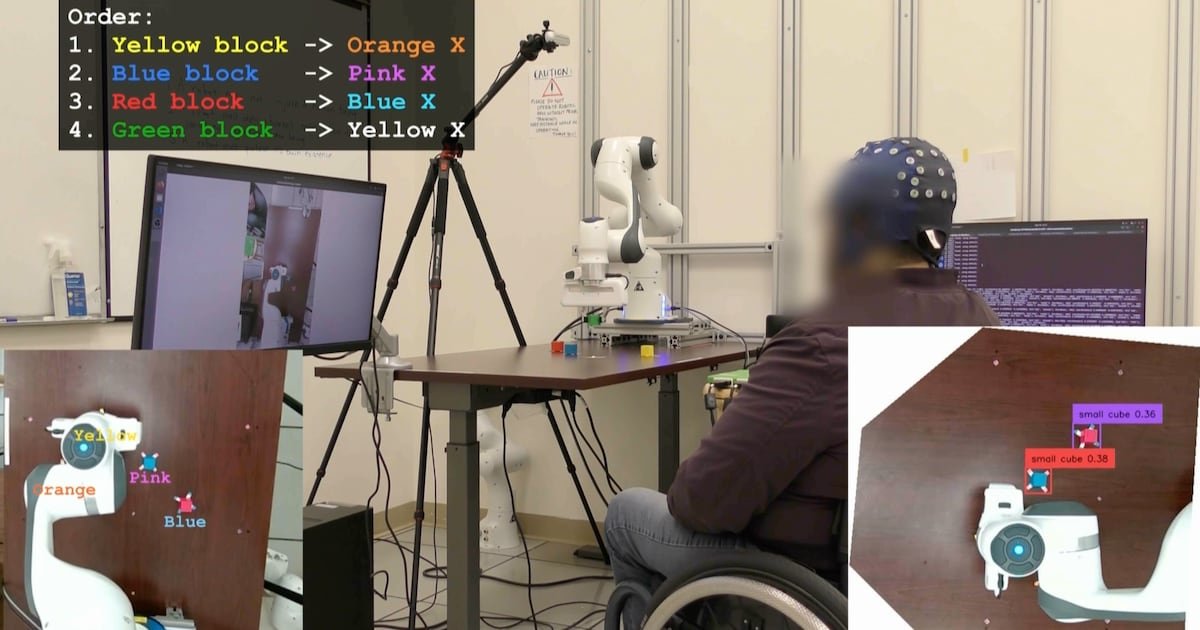
UCLA Researchers Enable Paralyzed Patients to Control Robots with Thoughts Using AI – CHOSUNBIZ – Chosun Biz
-

 Business3 days ago
Business3 days agoThe Guardian view on Trump and the Fed: independence is no substitute for accountability | Editorial
-
Tools & Platforms3 weeks ago
Building Trust in Military AI Starts with Opening the Black Box – War on the Rocks
-

 Ethics & Policy1 month ago
Ethics & Policy1 month agoSDAIA Supports Saudi Arabia’s Leadership in Shaping Global AI Ethics, Policy, and Research – وكالة الأنباء السعودية
-

 Events & Conferences3 months ago
Events & Conferences3 months agoJourney to 1000 models: Scaling Instagram’s recommendation system
-

 Jobs & Careers2 months ago
Jobs & Careers2 months agoMumbai-based Perplexity Alternative Has 60k+ Users Without Funding
-

 Funding & Business2 months ago
Funding & Business2 months agoKayak and Expedia race to build AI travel agents that turn social posts into itineraries
-

 Education2 months ago
Education2 months agoVEX Robotics launches AI-powered classroom robotics system
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoHappy 4th of July! 🎆 Made with Veo 3 in Gemini
-

 Podcasts & Talks2 months ago
Podcasts & Talks2 months agoOpenAI 🤝 @teamganassi
-

 Mergers & Acquisitions2 months ago
Mergers & Acquisitions2 months agoDonald Trump suggests US government review subsidies to Elon Musk’s companies